Predict the Pxy behavior for a mixture of pentafluo-roethane [R-125] (1) + isobutane (2) at 30C using
Question:
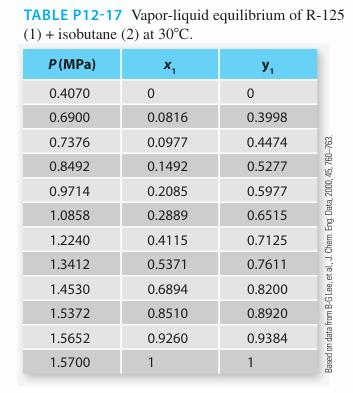
Predict the Pxy behavior for a mixture of pentafluo-roethane [R-125] (1) + isobutane (2) at 30°C using the Peng-Robinson equation of state. Compare the predicted values with experimental data given in Table P12-17. How would you suggest improving the modeling results from the Peng-Robinson equation relative to the experimental data?
Transcribed Image Text:
FF F-C-C-H II FF Pentafluoroethane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Predict the Pxy behavior for a mixture of cyclohexane (1) + 1-butanol (2) at 383.15 K using the Peng-Robinson equation of state. Compare the predictions to the experimental data given in Table...
-
Predict the Pxy behavior for a mixture of acetone (1) + 2-propanol (2) at 328.15 K using the Peng-Robinson equation of state. Compare the predictions to the experimental data given in Table P12-26....
-
Predict the Pxy behavior for a mixture of propane (1) + isobutane (2) at 30C using the Peng-Robinson equation of state. Com pare the predicted values with experimental data as given in Table P12-16....
-
A mixture of 1 kmol carbon dioxide, 2 kmol carbon monoxide, and 2 kmol oxygen, at 25C, 150 kPa, is heated in a constant pressure steady state process to 3000 K. Assuming that only these same...
-
Kowloon Trading Company, a wholly owned subsidiary incorporated in Hong Kong, imports macadamia nuts from its parent company in Honolulu for export to various duty-free shops in the Far East. During...
-
How do you know if a result is plausible? What techniques do you have to answer such questions?
-
In a single stage impulse turbine the blade angles are equal and the nozzle angle is \(20^{\circ}\). The velocity coefficient for the blade is 0.83. Find the mzximum blade efficiency possible. If the...
-
Dustin Clemens is the accounting and finance manager for a manufacturer. At year-end, he must determine how to account for the companys contingencies. His manager, Madeline Pretti, objects to...
-
Project teams can be made up of individuals from all over the country and around the world, with different time zones, cultures, work practices, languages, etc. In your role as a Project Manager,...
-
You need to determine the binary interaction parameter (k 12 ) for the Peng-Robinson equation of state for the tetra fluoromethane + trifluorochlo romethane system at 250 K and 225 K. The literature...
-
An equimolar mixture of methane and propane is discharged from a compressor at 5500 kPa and 90C at a rate of 1.4 kg/s. If the velocity in the discharge line is not to exceed 30 m/s, what is the...
-
Spreadsheet Problem. Refer to the Ch10_Mort Eq Cap tab in the Excel Workbook provided on the Web site. This replicates the example discussed on page 314 of the book. a. Suppose there is an aggressive...
-
1- Define and explain Business Process Remodelling? Why BPMN is important for businesses discuss with example (05 marks) 2-What is the principle of cardinality in date base modelling? Explain the...
-
give the Definitions/description of the following specialization structural engineering geotechnical engineering construction engineering transportation engineering water resources engineering
-
Taxable income Taxable income 0 - 50,000 50,001 - 75,000 75,001 - 100,000 100,001 - 335,000 335,001 - 10,000,000 10,000,001 - 15,000,000 15,000,001 - 18,333,333 18,333,334 + Output area: Taxes: 15%...
-
address each of the following questions: According to your log, to what type of media are you most exposed? Did this surprise you? Why or why not? If you could only access one form of media for the...
-
The end of year balances in the adjusted trial balance for Dynamic Weight Loss are as follows: Accounts Payable: $52,996 Accounts Receivable: $182,703 Accumulated Depreciation - Equipment: $173,182...
-
Natasha is a staff level auditor assigned to evaluate the ICFR for the XYZ corporation audit. Natasha follows her firms audit program to assess ICFR. Step 1.3 of the audit program says the auditor...
-
How do network effects help Facebook fend off smaller social-networking rivals? Could an online retailer doing half as much business compete on an equal footing with Amazon in terms of costs? Explain.
-
The array price given below contains the price in dollars of a certain stock over 10 days. Use MATLAB to determine how many days the price was above $20. price = [19, 18, 22, 21, 25, 19, 17, 21, 27,...
-
The arrays price_A and price_B given below contain the price in dollars of two stocks over 10 days. Use MATLAB to determine how many days the price of stock A was above the price of stock B. price_A...
-
The arrays price_A, price_B, and price_C given below contain the price in dollars of three stocks over 10 days. a. Use MATLAB to determine how many days the price of stock A was above both the price...
-
For the logic function f=ab+c Develop a Shannon's expansion theorem form of the logic function and sketch its implementation upon the CMOS logic cell shown in figure Q4b. XI XPolysilicon Z1 FZ2...
-
Using the following input data on HAM's portfolio for the month of October 2022, calculate the time-weighted rate of return for October. (3 marks) Market value 30 September 62,000,000 Cash...
-
3) Find a transformation 2x2 matrix A transform a vector 2x1 by reflect through the y-axis followed by a rotation clockwise 30, then reflect through the line --x 4) Given T:R'R' Find an equivalent...

Study smarter with the SolutionInn App


