Reactants A and B combine to form product P in the vapor phase reaction: A + B
Question:
Reactants A and B combine to form product P in the vapor phase reaction:
A + B ↔ P
But a side reaction also occurs, forming by-product U:
2A ↔ U
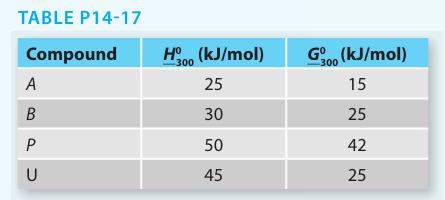
The Gibbs free energy and enthalpy of each compound at T = 300 K and P = 1 bar are given in Table P14-17. It is reasonable to assume that both reactions have ΔCP = 0 and that A, B, P, and U form ideal solutions.
These reactions are carried out in an isothermal batch reactor at a constant pressure of 1 bar. Find the contents of the reaction at equilibrium for each of the following.
A. The reactor is at T = 300 K and initially contains 10 moles each of A and B.
B. The reactor is at T = 500 K and initially contains 10 moles each of A and B.
C. The reactor is at T = 300 K and initially contains 15 moles of B and 5 moles of A.
D. The reactor is at T = 500 K and initially contains 15 moles of B and 5 moles of A.
E. The reactor is at T = 500 K and initially contains a total of 20 moles of reactants, A and B. What initial composition produces the maximum number of moles of P at equilibrium?
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





