We know that many binary mixtures contain an azeotrope, which means that the liquid and vapor phases
Question:
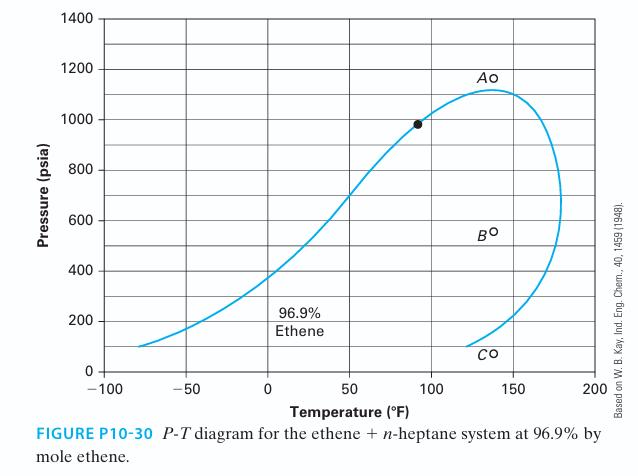
We know that many binary mixtures contain an azeotrope, which means that the liquid and vapor phases for the mixture have the same composition. Can a system have two azeotropes for a given pressure or temperature? If it exists, find a system and plot the data.
Transcribed Image Text:
Pressure (psia) 1400 1200 1000 800 600 400 200 0+ -100 -50 0 96.9% Ethene 100 Ao BO 50 Temperature (°F) FIGURE P10-30 P-T diagram for the ethene + n-heptane system at 96.9% by mole ethene. со 150 200 Based on W. B. Kay, Ind. Eng. Chem., 40, 1459 (1948).
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
Vapor?liquid equilibrium data for mixtures of acetone (A) and ethanol at 1 atm are given in the following table: (a) Use the given data to construct a Txy diagram for this system. (b) A thermocouple...
-
A binary mixture of mole fraction zj is flashed (o conditions T and P, Fur one of the following determine: the equilibrium mole fractions x1 and y1 of the liquid and vapor phases formed, the molar...
-
Q8 Question: 9 A small particle of mass m moving inside a heavy, hollow and straight tube along the tube axis undergoes elastic collision at two ends. The tube has no friction and it is closed at one...
-
Jody Hunter and Jim Boling, two managers at Georgia- Pacific Corporation, a paper manufacturer, disagree about continuing the companys membership in Business for Affordable Medicine (BAM), a...
-
If we define the median of a sequence as a number so that exactly as many elements come before it in the sequence as come after it, fix the program in 4.6.3 so that it always prints out a median....
-
What are the requirements of a modern surface condenser ?
-
a. Cal Ruther, an employer, is subject to FICA taxes but exempt from FUTA and SUTA taxes. During the last quarter of the year, his employees earned monthly wages of $8,500, all of which is taxable....
-
Huegel Hollow Resort has ordered 24 rotomolded kayaks from Current Designs. Each kayak will be formed in the rotomolded oven, cooled, and then have the excess plastic trimmed away. Then, the hatches,...
-
Figure P10-30 is a P-T diagram for the ethene + n-heptane system at 96.9% by mole ethene (Kay, 1948). Please answer the following questions based on this figure. Note that the in the figure...
-
A separation stream off the main reactor effluent contains almost exclusively ethyl benzene, benzene, and toluene at 1 bar and 100C. You determine that the stream flow rate is made up of 34 kg/s of...
-
A tired worker pushes with a horizontal force of 500 N on a 100-kg crate resting on a thick pile carpet. The coefficients of static and kinetic friction are 0.6 and 0.4, respectively. Find the...
-
An agent with wealth level Yo = $100 lives for two periods. In period 1, he invests $20 in a risk free bond, consumes some of his wealth and invests the rest of his wealth in the stock market...
-
1. For the following cable system, the sag at B and D is 4 ft. Determine the tension at the support E and the maximum sag. A Ax Ay 4 m 10' 5 -3 m B hB 3 kN 10 B hc 10' 300# 2kN ho 300# (Source:...
-
At the beginning of January 2020, the ledger of Powermix Company showed Cash of P750,000 and R. Manalo Capital of P750,000. Powermix completed the following transactions in the month of January:...
-
After several years of designing and selling her own stationery, Susan has decided to expand her stationery company by moving into a large building and hiring staff. Operating under the banner of...
-
Assume the cost of equity is 10.18%; the dividend growth rate is 3.50% in the perpetuity and that WACC is 8.14%. Use the income approach and value the shares of the subject company as at the end of...
-
What management responsibility is established by Section 302 of SOX?
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
The mechanical work W done in using a force F to push a block through a distance D is W ! FD. The following table gives data on the amount of force used to push a block through the given distance...
-
Plane A is heading southwest at 300 mi/hr, while plane B is heading west at 150 mi/hr. What are the velocity and the speed of plane A relative to plane B?
-
The following table shows the hourly wages, hours worked, and output (number of widgets produced) in one week for ve widget makers. Use MATLAB to answer these questions: a. How much did each worker...
-
Identify two key client objectives Work with the person to identify actions and activities to support these objectives. When discussing objectives and actions and activities, ensure that you are...
-
Give the journal entry and the impact on total equity for each of the following (independent) transactions: Declaration and payment of cash dividend of $3,000. 50% stock dividend. At the time of the...
-
What was the cash received for interest by the following company in 2 0 2 5 ? Use the information provided below. Type your answer using numbers only - no dollar signs and no commas. The income...

Study smarter with the SolutionInn App


