(A) Calculate the quantity of that would be obtained if suggestions (1) and (2) in Example 14-4(b)...
Question:
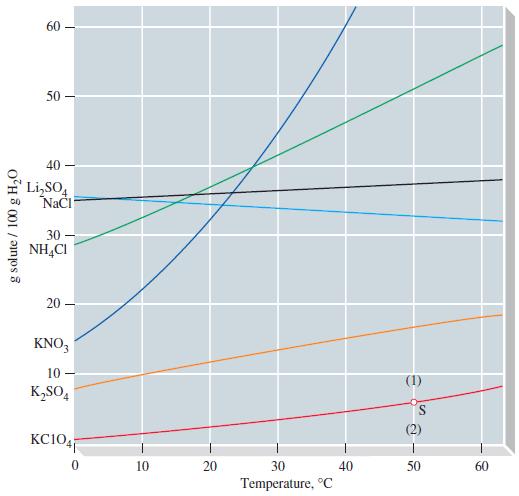
(A) Calculate the quantity of that would be obtained if suggestions (1) and (2) in Example 14-4(b) were followed. Use data from Figure 14-10. What mass of water is needed to produce a saturated solution containing 95 g NH4Cl at 60 °C?
(B) Use Figure 14-10 to examine the solubility curves for the three potassium salts: KClO4, K2SO4, and KNO3. If saturated solutions of these salts at 40 °C are cooled to 20 °C rank the salts in order of highest percent yield for the recrystallization.
Example 14-4(b)
(b) How might we improve the yield of NH4Cl?
Figure 14-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





