A lithium battery, uses lithium metal as one electrode and carbon in contact with MnO 2 in
Question:
A lithium battery, uses lithium metal as one electrode and carbon in contact with MnO2 in a paste of KOH as the other electrode. The electrolyte is lithium perchlorate in a nonaqueous solvent, and the construction is similar to the silver battery. The half-cell reactions involve the oxidation of lithium and the reaction
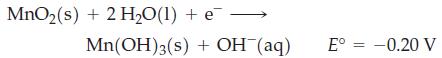
Draw a cell diagram for the lithium battery, identify the negative and positive electrodes, and estimate the cell potential under standard conditions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





