A solution of 20.0 g KClO 4 in 500.0 g of water is brought to a temperature
Question:
A solution of 20.0 g KClO4 in 500.0 g of water is brought to a temperature of 40 °C.
(a) Refer to Figure 14-10 and determine whether the solution is unsaturated or supersaturated at 40 °C.
(b) Approximately what mass of KClO4, in grams, must be added to saturate the solution (if originally unsaturated), or what mass of KClO4 can be crystallized (if originally supersaturated)?
Figure 14-10
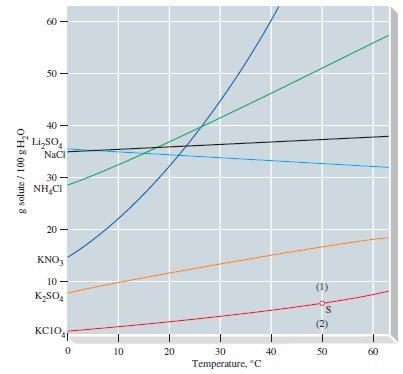
Transcribed Image Text:
g solute / 100 g H₂0 8 50- Li₂SO NaCl 30- NHẠCI 20 KNO3 10- K₂SO4 wow KCIO 0 10 20 30 Temperature, C 40 30-8 50 60
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a According to Figure 1410 the solubility of KClO4 in water at 40 C is approximately 46 g100 g H2O S...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In mining engineering, holes are often drilled through rock, using drill bits. As a drill hole gets deeper, additional rods are added to the drill bit to enable additional drilling to take place. It...
-
In mining engineering, holes are often drilled through rock, using drill bits. As a drill hole gets deeper, additional rods are added to the drill bit to enable additional drilling to take place. It...
-
In mining engineering, holes are often drilled through rock, using drill bits. As a drill hole gets deeper, additional rods are added to the drill bit to enable additional drilling to take place. It...
-
State whether the following statement are True or False An increase in the owner(s) equity, in the absence of any further investment by the owners is typically effected by sale transactions.
-
Using the information in P11-2A, compute the overhead controllable variance and the overhead volume variance. Data From P11-2A, Ayala Corporation accumulates the following data relative to jobs...
-
If the resultant force F R is directed along a line measured 75? clockwise from the positive x axis and the magnitude of F 2 is to be a minimum, determine the magnitudes of F R and F 2 and the angle...
-
Sometimes people who act inappropriately are just trying to save their jobs and the company. How do you feel about those types of persons?
-
Peoria Corp. just completed another successful year, as indicated by the following income statement: Presented here are comparative balance sheets: Other information is as follows: a. Dividends of...
-
1. A thin film is laid over a glass pane as shown. White light is incident on the film, coming straight in. At a point where the light is incident on the film, it appears green ( = 525 nm). Find (a)...
-
One way to recrystallize a solute from a solution is to change the temperature. Another way is to evaporate solvent from the solution. A 335 g sample of a saturated solution of KNO 3 (s) in water is...
-
Refer to Figure 14-10 and estimate the temperature at which a saturated aqueous solution of KClO 4 is 0.200 m. Figure 14-10 g solute / 100 g H0 8 50- LiSO NaCl 30- NHCI 20 KNO3 10- KSO4 wow KCIO 0...
-
In Problems 6570, discuss the validity of each statement. If the statement is always true, explain why. If not, give a counterexample. If C is a state in the transition diagram for a Markov chain,...
-
Clemens County had the following revenue sources in 20XS: Required Prepare a schedule computing the amount of general revenues and of program revenues that Clemens County should report in its...
-
Dorrian Countys fund structure is as follows: Required a. What column headings would the county need to present in its governmental funds Statement of Revenues, Expenditures, and Changes in Fund...
-
The following information was drawn from the accounts and records of Mosser Township: Prepare a schedule computing the amounts to be reported in each of the three minimum program revenues...
-
Prepare a Statement of Activities for Tazewell County for calendar year 20X9, given the following: General property tax revenues..... Proceeds from sale of general government land* Unrestricted grant...
-
Presented here is the preclosing trial balance information for the total of Locklear Countys four Enterprise Funds for the year ended September 30, 20X7. 1. The Contribution from Locklear County...
-
For each separate case below, follow the 3-step process for adjusting the accumulated depreciation account: Step 1: Determine what the current account balance equals. Step 2: Determine what the...
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
The first floating-rate preferreds were successfully issued at initial yields below yields on Treasury bills. How was this possible? The preferreds were clearly riskier than the bills. What would you...
-
Listed below are some common terms of sale. Can you explain what each means? a. 2/30, net 60. b. net 10. c. 2/5, EOM, net 30.
-
Some of the items in question 1 involve a cash discount. For each of these, calculate the rate of interest paid by customers who pay on the due date instead of taking the cash discount.
-
The Canadian Restaurant Association states that the restaurant industry has an economic effect of more than $1.7 trillion annually, with every dollar spent in restaurants generating an estimated...
-
O O A Dundas Company's inventory records for its retail division show the following at May 31: (Click the icon to view the accounting records.) At May 31, 10 of these units are on hand. Dundas...
-
4. R got 35% hike in his salary and 20% incentive on sales. If R sold goods worth Rs. 850 last year and the salary was Rs 70, then how much more does he earn this year with sales of Rs.900?

Study smarter with the SolutionInn App


