(a) Use the value of the van der Waals constant b for CH 4 (g), given in...
Question:
(a) Use the value of the van der Waals constant b for CH4(g), given in Table 6.5, to estimate the radius of the CH4 molecule. (See Exercise 89.) How does your estimate of the radius compare with the value r = 228 pm, obtained experimentally from an analysis of the structure of solid methane?
(b) The density of CH4(g) is 66.02 g mL-1 at 100 bar and 325 K. What is the value of the compressibility factor at this temperature and pressure?
Exercise 89
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers.
Table 6.5
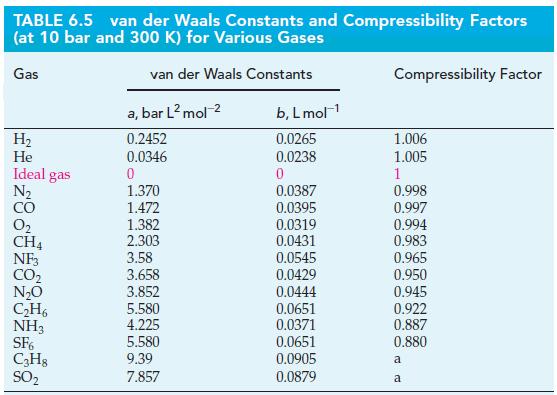
TABLE 6.5 van der Waals Constants and Compressibility Factors (at 10 bar and 300 K) for Various Gases van der Waals Constants Gas H₂ He Ideal gas N₂ CO 0₂ CH4 NF3 CO₂ N₂O C₂H6 NH3 SF6 C3H8 SO₂ a, bar L² mol-² 0.2452 0.0346 0 1.370 1.472 1.382 2.303 3.58 3.658 3.852 5.580 4.225 5.580 9.39 7.857 b, L mol-¹ 0.0265 0.0238 0 0.0387 0.0395 0.0319 0.0431 0.0545 0.0429 0.0444 0.0651 0.0371 0.0651 0.0905 0.0879 Compressibility Factor 1.006 1.005 1 0.998 0.997 0.994 0.983 0.965 0.950 0.945 0.922 0.887 0.880 a a
Step by Step Answer:
The van der Waals equation for a real gas is given by P Vb RT where P is the pressure V is the molar ...View the full answer



General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Students also viewed these Sciences questions
-
Use the value of the van der Waals constant b for He(g) given in Table 6.5, to estimate the radius, r, of a single helium atom. Give your answer in picometers. Table 6.5 TABLE 6.5 van der Waals...
-
A certain gas obeys the van der Waals equation with a =0.76 m6 Pa mol-2, its volume is found to be 4.00 X 10-4 m3 mol-1 at 288 K and 4.0 MPa. From this information calculate the van der Waals...
-
The volume of a spherical molecule can be estimated as V = b/(4N A ), where b is the van der Waals parameter for the excluded molar volume and N A is Avogadros number. Justify this relationship by...
-
If you uncover critically important information (the sort that could make or break your company) that is from a credible source and appears to be unbiased, well documented, current, and complete but...
-
In addition to your regular responsibilities, your supervisor has just assigned you to be in charge of your organizations annual golf tournament. It is expected that 100 to 150 employees will enter...
-
AEK Ltd has prepared its income statement, summarised below, for the year ended 30 June 2025. The company is evaluating three independent situations and has asked for your assistance. Required (a) If...
-
\(C_{P}=C_{V}\) when (a) \(\left(\frac{\partial V}{\partial T} ight)_{P}=0\) (b) \(\left(\frac{\partial V}{\partial P} ight)_{T}=0\) (c) \(\left(\frac{\partial P}{\partial T} ight)_{V}=0\) (d) None...
-
Two aqueous sulfuric acid solutions containing 20.0 wt% H 2 SO 4 (SG = 1.139) and 60.0 wt% H 2 SO 4 (SG = 1.498) are mixed to form a 4.00 molar solution (SG = 1.213). (a) Calculate the mass fraction...
-
During the year, TRC Corporation has the following inventory transactions. Date January 1 Transaction Beginning inventory April 7 Purchase July 16 Purchase October 6 Purchase Number of Unit Total...
-
Assume the following initial conditions for the graphs labeled A, B, and C in Figure 6-7. (A) 10.0 mL at 400 K; (B) 20.0 mL at 400 K; (C) 40.0 mL at 400 K. Use Charless law to calculate the volume of...
-
Refer to Example 6-17. Recalculate the pressure of Cl 2 (g) by using both the ideal gas equation and the van der Waals equation at the temperatures (a) 100 C; (b) 200 C; (c) 400 C. From the results,...
-
World Systems manufactures an optical switch that it uses in its final product. World Systems incurred the following manufacturing costs when it produced 66,000 units last year: Direct materials . ....
-
Complete the code below to sum all the numbers in all inner arrays and print the result in a new 1
-
Write a program that finds the largest in a series of numbers entered by the user. The pro- gram must prompt the user to enter numbers one by one. When the user enters 0 or a nega- tive number, the...
-
By watching the expression Mynum-4 mynum + c from a programming language, what can we say about this language? This is not an imperative programming language This is a case sensitive language This is...
-
Consider the Markov Chain, Xn, on the states i = 0, 1, 2, . . . with transition matrix given by pi,i1 = p i = 1, 2, . . . pi,i+1 = 1 p i = 0, 1, . . . p0,0 = p where 0 < p < 1. (i) Show that the...
-
For each firm, calculate the return on equity (ROE), the dividend per share, and the earnings per share for the five years. Record them in a spreadsheet. b. Calculate the dividend payout ratio...
-
How do the ABC categories (hierarchy) in a service company differ from those in a manufacturing company?
-
Consider the advantages and disadvantages of extending property rights so that everyone would have the right to prevent people imposing any costs on them whatsoever (or charging them to do so).
-
Explain how a non-consolidated subsidiary can be a form of off-balance-sheet financing.
-
Where can authoritative i GAAP guidance related to liabilities are found?
-
Briefly describe some of the similarities and differences between U.S. GAAP and iGAAP with respect to the accounting for liabilities.
-
Cullumber Company is considering a long-term investment project called ZIP. ZIP will require an investment of $104,000. It will have a useful life of 4 years and no salvage value. Annual revenues...
-
Pretax financial statement income for the year ended December 31, 2024 was $140 million for Walters Company. Walters's taxable income was $100 million. This was a result of differences between...
-
Several years ago, Cyclop Company issued bonds with a face value of $1,000,000 for $1,130,000. As a result of declining interest rates, the company has decided to call the bonds at a call premium of...

Study smarter with the SolutionInn App


