(A) Write thermodynamic equilibrium constant expressions for each of the following reactions. Relate these to K c...
Question:
(A) Write thermodynamic equilibrium constant expressions for each of the following reactions. Relate these to Kc or Kp where appropriate.
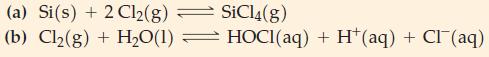
(B) Write a thermodynamic equilibrium constant expression to represent the reaction of solid lead(II) sulfide with aqueous nitric acid to produce solid sulfur, a solution of lead(II) nitrate, and nitrogen monoxide gas. Base the expression on the balanced net ionic equation for the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





