Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard
Question:
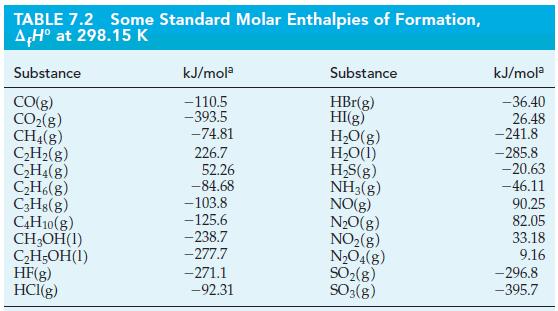
Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard enthalpy of combustion for formic acid (ΔrH° = -255 kJ/mol) to calculate the standard enthalpy of formation for formic acid.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(8) C₂H₂(g) C₂H4(8) C₂H6(g) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The standard enthalpy change for the combustion of formic acid can be represented as follows HCOOHI ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy of combustion of ethene gas [C2H4(g)] is 1411.1 kJ/ mol at 298 K. Given the following enthalpies of formation, calculate Hof for C2H4(g). CO2(g) 393.5 kJ/ mol H2O(l) 285.8 kJ/...
-
The standard enthalpy of combustion of cyclopropane is 2091 kJ mol 1 at 25C. From this information and enthalpy of formation data for CO 2 (g) and H 2 O(g), calculate the enthalpy of formation of...
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
Consider the following types of images: i. Real, inverted and highly diminished image. ii. Real, inverted and enlarged image. iii. Virtual, erect and enlarged image. iv. Virtual, erect and diminished...
-
Managerial accounting is more than recording, maintaining, and reporting financial results. Managerial accountants must provide managers with both financial and nonfinancial information including...
-
1. Howard City should use a capital projects fund to account for: a. Proceeds of a capital grant to finance a new civic center that will not provide services primarily on a user-charge basis b....
-
Use the data in Exercise 26 in Section 13.1 for the following. a. Compute a point estimate for the mean evaporation rate when the temperature is 20C. b. Construct a 99% confidence interval for the...
-
EKC Company uses the retail inventory method. The following information for 2016 is available: Required: Compute the cost of the ending inventors under each of the following cost flow assumptions...
-
Describe the dividend policy of Berkshire Hathaway has adopted. Consider the historical dividend payments and the company's public statements regarding dividend distribution. (can you write a...
-
On December 1, 2020, Papadopoulos Seasonings had the following account balances. During December, the company completed the following transactions. Dec. 7 Received 3,600 cash from customers in...
-
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH 3 CH(OH)COOH(s)]: f H = -694.0 kJ/mol. Table 7.2 TABLE...
-
The decomposition of limestone, CaCO 3 (s), into quicklime, CaO(s), and CO 2 (g) is carried out in a gas-fired kiln. Use data from Appendix D to determine how much heat is required to decompose 1.35...
-
Air at \(4 \times 10^{-4} \mathrm{~kg} / \mathrm{s}\) and \(20^{\circ} \mathrm{C}\) enters a rectangular duct that is \(1 \mathrm{~m}\) long and \(4 \mathrm{~mm} \times 16 \mathrm{~mm}\) on a side. A...
-
What is the difference between global, patchwork, and incremental intellectual theft?
-
Describe the four most common types of investment companies.
-
What are the various types of slides you might use as presentation aids? What kind of data is each designed to present?
-
Why do effective speakers moderate their gestures?
-
Besides having a claim on income what is the most important right of common stockholders? Why is this right important?
-
Briefly describe several types of information that are especially well suited to publication on the Internet. What are the differences between the online and print versions, and when would you use...
-
Which internal control principle is especially diffi cult for small organizations to implement? Why?
-
Capitalization of Interest on July 31, 2010, Bismarck Company engaged Duval Tooling Company to construct a special-purpose piece of factory machinery. Construction was begun immediately and was...
-
Capitalization of Interest the following three situations involve the capitalization of interest. Situation I On January 1, 2010, Columbia, Inc. signed a fixed-price contract to have Builder...
-
Entries for Equipment Acquisitions Chopin Engineering Corporation purchased conveyor equipment with a list price of $15,000. Presented below are three independent cases related to the equipment....
-
The amount of R 2 5 0 DR EFT appeared on the bank statement but did not appear on the CRJ or CPJ . It was outstanding. Also a deposit of R 7 0 0 appeared on the credit side of bank statement but not...
-
The True State of the US Economy. https://tcf.org/content/report/true-state-u-s-economy/?gclid= EAIaIQobChMIm9Cavfyf_AIVwIJaBR2VLwg3EAAYASAAEgJ6LvD_BwE Question 1.- What are the social causes and...
-
Imagine that you are playing the "ultimatum game". The other player (whom you cannot observe) is of your same economic and social group. The sum of money to be divided in the game is $1,000. a. If...

Study smarter with the SolutionInn App


