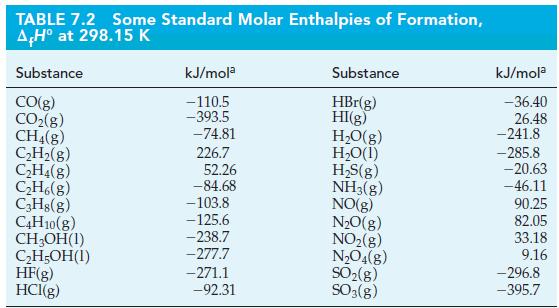
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the
Question:
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH3CH(OH)COOH(s)]: ΔfH° = -694.0 kJ/mol.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(g) C₂H₂(g) C₂H4(g) C₂H6(g) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCI(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(8) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The enthalpy of combustion AH combustion for lactic acid CH3CHOHCOOH can be calculated using the sta...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Create a class Restaurant with the following attributes: restaurant_ld, name, address, where the restaurant_ld is a unique identifier of type 'int', name is of type 'str' and address is of type...
-
Calculate the enthalpy of combustion (hoC) of gaseous methane, in kJ per kg of fuel, (a) At 25oC, 100 kPa with water vapor in the products, (b) At 25oC, 100 kPa with liquid water in the products.
-
The standard enthalpy of combustion of cyclopropane is 2091 kJ mol 1 at 25C. From this information and enthalpy of formation data for CO 2 (g) and H 2 O(g), calculate the enthalpy of formation of...
-
Which of the following do ergonomics most directly address? Physical wellbeing Mental wellbeing Emotional wellbeing Social wellbeing
-
Best Buy and RadioShack are both merchandisers that rely on customer satisfaction. Access and read (1) Best Buys Business Strategy and Core Philosophies section (one page) and (2) RadioShacks...
-
(a) Calculate the current ratio if current assets are 60,000 and current liabilities are 50,000. (b) If inventory is 32,000, what is the acid test ratio?
-
Petitioner Salman was indicted for federal securities-fraud crimes for trading on inside information he received from a friend and relative-by-marriage, Michael Kara, who, in turn, had received the...
-
For the following independent situations, assume that you are the audit partner on the engagement: 1. In the last 3 months of the current year, Oil Refining Company decided to change direction and go...
-
Tl-208 decays through a beta emission and has a half-life of 3.05 minutes. Besides the beta emission, there are several gamma rays associated with the decay and these gamma emissions occur at...
-
Health Valley Hospital provides comprehensive services, including cancer, heart, trauma, and emergency services. It has 2,300 full-time employees. For eight years, Health Valley has had a...
-
What volume of 18.5 C water must be added, together with a 1.23 kg piece of iron at 68.5 C, so that the temperature of the water in the insulated container shown in the figure remains constant at...
-
Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard enthalpy of combustion for formic acid ( r H = -255 kJ/mol) to calculate the standard enthalpy of formation...
-
How does the role of the FASB differ from that of the Securities and Exchange Commission with regard to the establishment of accounting standards?
-
Renata (20, not blind) is a student and can be claimed as a dependent by her uncle. She is single. Her income is unearned income of $212 and earned income of $12,721 in tax year 2023. Is Renata...
-
Jan pays for her qualified long term care policy via a health savings account. What amount of her premium payment would be taxable as ordinary income upon withdrawal?
-
A small company is limited in its ability to hire enough people in accounting to prevent an employee from being able to both steal money and cover up the theft in the accounting records. To which...
-
When reporting to your supervisor you can provide the report in either verbal or written form depending on the situation. List two (2) methods for verbal reporting and two (2) methods for written...
-
Guillermo gets a speeding ticket from Officer Speedtrap and hires Natascha, a licenced paralegal to defend him in court. They appear in front of Judge Friendly in provincial offences court. Who is...
-
ExxonMobil Corporation had net income of $41.0 billion in 2011. Inventories under the LIFO method used by the company were $11.7 billion in 2011. Inventory would have been $25.6 billion higher if the...
-
Feller Company purchased a site for a limestone quarry for $100,000 on January 2, 2019. It estimate that the quarry will yield 400,000 tons of limestone. It estimates that its retirement obligation...
-
Capitalization of Interest McPherson Furniture Company started construction of a combination office and warehouse building for its own use at an estimated cost of $5,000,000 on January 1, 2010....
-
Capitalization of Interest McPherson Furniture Company started construction of a combination office and warehouse building for its own use at an estimated cost of $5,000,000 on January 1, 2010....
-
Capitalization of Interest On December 31, 2009, Hurston Inc. borrowed $3,000,000 at 12% payable annually to finance the construction of a new building. In 2010, the company made the following...
-
Precision Cuts has a target debt-equity ratio of .48. Its cost of equity is 16.4 percent, and its pretax cost of debt is 8.2 percent. If the tax rate is 34 percent, what is the company's WACC?
-
Traditional Bank has an issue of preferred stock with an annual dividend of $7.50 that just sold for $62 a share. What is the bank's cost of preferred stock?
-
A 230g ball is dropped from a height of 1.9m and bounces on a hard floor. The force on the ball from the floor is shown in the figure. How high does the ball rebound? F, (N) y 1000 0- -t (ms) 02 4 6...

Study smarter with the SolutionInn App


