Concerning the electrons in the shells, subshells, and orbitals of an atom, how many can have mg
Question:
Concerning the electrons in the shells, subshells, and orbitals of an atom, how many can have
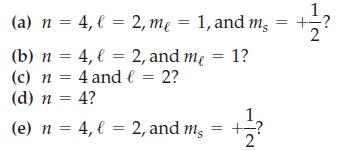
Transcribed Image Text:
mg 1, and m² = +- ? (a) n = 4, = 2, me = 1, and (b) n = 4, l = 2, and me = 1? (c) n = 4 and l = 2? (d) n = 4? 1 (e) n = 4, l = 2, and m, = +=?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a For n 4 l 2 and ml 1 there are two possible values for ms 12 and 12 Therefore there are ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(a) If the valence atomic orbitals of an atom are sp hybridized, how many unhybridized p orbitals remain in the valence shell? How many bonds can the atom form? (b) Imagine that you could hold two...
-
How many subshells are completely filled with electrons for Na? How many subshells are unfilled?
-
How many subshells are completely filled with electrons for Mg? How many subshells are unfilled?
-
Define the necessary and sufficient condition for two transactions to be serialisable. Give an example of a non-serialisable execution of a pair of transactions. [3 marks] (c) Define the necessary...
-
1. Why do you think Starbucks has been so concerned with social responsibility in its overall corporate strategy? 2. Is Starbucks unique in being able to provide a high level of benefits to its...
-
In an effort to conserve energy in a heat-engine cycle, somebody suggests incorporating a refrigerator that will absorb some of the waste energy QL and transfer it to the energy source of the heat...
-
A large company has the opportunity to select one of seven projects-A, B, C, D, E, F, G-or choose the null (donothing) alternative. Each project requires a single initial investment as shown in the...
-
Perform analytical procedures for accounts payable of J & J Auto Repair Service in the following manner: a. Calculate and list all necessary figures and comparisons. b. Explain what the result of...
-
There are two primary reasons that guide my approach to back up files externally. First, there's the aspect of cost savings. Investing in a hard drive with sufficient storage capacity constitutes a...
-
1- The outflow concentration from a reactor is measured at a number of times over a 24-hr period: t, hr 5.5 10 12 14 16 18 20 24 C, mg/ 1.5 2.3 2.1 4 5 5.5 5 3 1.2 The flow rate for the outflow in...
-
Which of the following statements is (are) correct for an electron with n = 4 and m = 2? Explain. (a) The electron is in the fourth principal shell. (b) The electron may be in a d orbital. (c) The...
-
What type of orbital (i.e., 3s, 4p, . . . ) is designated by these quantum numbers? (a) n = 5, l = 1, me = 0 (b) n = 4, l = 2, me = -2 (c) n = 2, l = 0, me = 0
-
Consider Figure 4.11, which shows the sensitivity report for the Flair Furniture example in Section 4.3. For each of the situations described below, check to see if the 100% rule can be used to...
-
Prepare a physical unit flow reconciliation with the following information. Blending Process Units of Product Beginning work in process inventory 168,000 Units started this period 355,000 Units...
-
What is the value of 'm'? int x=1; int y = 2; int z=3; int m = x+y+z; if (x
-
2. Write a program that implements the following arithmetic expression: EAX = -val2 + 7 val3 + vall - Use the following data definitions: vall SDWORD 8 va12 SDWORD -15 va13 SDWORD 20 In comments next...
-
Baskin-Robbins is one of the worlds largest specialty ice cream shops. The company offers dozens of different flavors, from Very Berry Strawberry to lowfat Espresso n Cream. Assume that a local...
-
We ran hundreds of different applications and generated the following table: Instruction Type ALU Load Store Branch Frequency of use (%) Cycles to Execute 50% 1 30% 4 10% 10% 3 10 A chief designer...
-
The units of an item available for sale during the year were as follows: There are 57 units of the item in the physical inventory at December 31. The periodic inventory system is used. Determine the...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Why do employees typically want more authority and less responsibility?
-
Consider the job you now hold or one that you held in the past. Did your boss have the authority to direct your work? Why did he or she have this authority?
-
Describe at least four features of organization structure that were important parts of the classic view of organizing.
-
What role does psychological contract theory play in understanding the reciprocal expectations between principals and agents in delegated relationships, and how can discrepancies in these contracts...
-
Persuasive Slide Deck Scenario You work for Jake Zucker Consulting, a business consulting company that focuses on providing reports to its clients on new trends that may have an impact on them....
-
. what is the concept of supply chain surplus?

Study smarter with the SolutionInn App


