Determine the molar solubility of lead(II) azide, Pb(N 3 ) 2 , in a buffer solution with
Question:
Determine the molar solubility of lead(II) azide, Pb(N3)2, in a buffer solution with pH = 3.00, given that
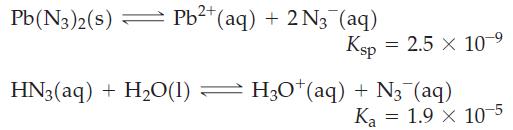
Transcribed Image Text:
Pb(N3)2(s) Pb²+ (aq) + 2N3 (aq) = Ksp 2.5 x 10 ⁹ HN3(aq) + H₂O(1) — H3O*(aq) + N₂ (aq) = Ka 1.9 x 10-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the molar solubility of leadII azide PbN32 in a buffer solution with pH 300 we need ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A saturated solution of lead iodate in pure water has an iodate-ion concentration of 8.0 10-5 M. a. What is the molar solubility of lead iodate in a 0.15 M lead nitrate solution at the same...
-
The solubility of CaCO 3 is pH dependent. a) Calculate the molar solubility of CaCO 3 (K sp = 4.5 10 -9 ) neglecting the acidbase character of the carbonate ion. (b) Use the expression for the CO 3...
-
The K sp of Ca 3 (PO 4 ) 2 is 2.0 10 33 . Determine its molar solubility in 0.05 M (NH 4 ) 3 PO 4 . Compare your answer to the molar solubility of Ca3(PO4)2 in water, which we calculated in Example...
-
Using the framework of the marketing mix, appraise the marketing tactics of Boo.com in the areas of Product, Pricing, Place, Promotion, Process, People and Physical Evidence.
-
Transistor Electronics makes all of its sales on credit and accounts for them using the installment sales method. For simplicity, assume that all sales occur on the first day of the year and that all...
-
Explain the three reasons the aggregate-demand curve slopes downward give an example of an event that would shift the aggregate-demand curve. Which way would this event shift the curve?
-
Consider the following cash flow profile and assume MARR is 10 percent/year. a. What does Descartes' rule of signs tell us about the IRR(s) of this project? b. What does Norstrom's criterion tell us...
-
Stephen King, D.D.S., opened a dental practice on January 1, 2012. During the first month of operations, the following transactions occurred. 1. Performed services for patients who had dental plan...
-
From the following ER diagram a) Translate it into a relational model b) Normalize it into BCNF (if possible) user id email name item_id description AuctionStartDate password phone_no age Browses 1...
-
Assume that the seawater sample described in Example 18-6 contains approximately 440 g Ca 2+ per metric ton (1 metricton = 10 3 kg; density of seawater = 1.03 g/mL) (a) Should Ca(OH) 2 (s)...
-
Calculate the molar solubility of Mg(OH) 2 in 1.00 M NH 4 Cl(aq).
-
Chevalier de Mr used to bet that he could get at least one 6 in four rolls of a die. He also bet that, in 24 tosses of a pair of dice, he would get at least one 12. He found that he won more often...
-
The capital structure of a publicly traded company contains a single debt issue with the following characteristics: Fixed coupon-paying bond issue (semiannual pay) Par value: $80 million Coupon rate:...
-
A regulatory specialist may be tasked with presenting regulatory information on provider and patient rights in a way that all department employees and patients will understand the importance of...
-
The graph to the right depicts an economy, Home, which produces flowers and soybeans. Its production possibilities frontier is shown as TT. One of Home's isovalue lines is also shown as VV. Home...
-
Required: Calculate the covariance Invest 50% of your money in Asset A and 50% in B State of the economy Boom Bust Probability A return B return 0,4 20% 15% 0,6 -10% 9%
-
Being able to identify, create, and mess up arguments teaches so many useful lessons in thinking that we're going to give it the time it needs. Please answer the following questions fully and...
-
What are the major lessor groups? What advantage does a captive have in a leasing arrangement?
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
Tony Masasi started his own consulting firm, Masasi Company, on June 1, 2010. The trial balance at June 30 is shown below. In addition to those accounts listed on the trial balance, the chart of...
-
Neosho River Resort opened for business on June 1 with eight air-conditioned units. Its trial balance before adjustment on August 31 is as follows. In addition to those accounts listed on the trial...
-
Fernetti Advertising Agency was founded by John Fernetti in January of 2009. Presented on page 134 are both the adjusted and unadjusted trial balances as of December 31, 2010. Instructions(a)...
-
P&G Segments Its Market Along Multiple Dimensions 1. In addition to the list of options presented in this case, how else might a multibrand conglomerate like P&G segment its millions of customers?...
-
How can companies effectively utilize social media platforms to enhance brand awareness and engage with their target audience?
-
Evaluate /4 tan(0) sec (0)de

Study smarter with the SolutionInn App


