For LiBr, the dipole moment (measured in the gas phase) and the bond length (measured in the
Question:
For LiBr, the dipole moment (measured in the gas phase) and the bond length (measured in the solid state) are 7.268 D and 217 pm, respectively. For NaCl, the corresponding values are 9.001 D and 236.1 pm.
(a) Calculate the percent ionic character for each bond.
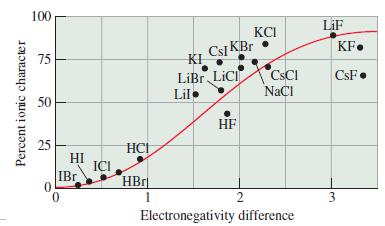
(b) Compare these values with the expected ionic character based on differences in electronegativity (see Figure 10-7).
(c) Account for any differences in the values obtained in these two different ways.
Figure 10-7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





