For the voltaic cell, (a) what isE cell initially? (b) As the cell operates, willE cell increase,
Question:
For the voltaic cell,
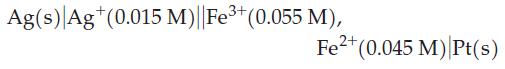
(a) what is Ecell initially?
(b) As the cell operates, will Ecell increase, decrease, or remain constant with time? Explain.
(c) What will be Ecell when [Ag+] has increased to 0.020 M?
(d) What will be [Ag+] when Ecell = 0.010 V?
(e) What are the ion concentrations when Ecell = 0?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





