For which of the following reactions would you expect the extent of the forward reaction to increase
Question:
For which of the following reactions would you expect the extent of the forward reaction to increase with increasing temperatures? Explain.
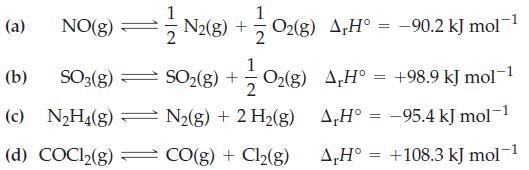
Transcribed Image Text:
(a) NO(g) =// N2(8) + O₂(8) A‚H° = −90.2 KJ mol¯ O₂(g) A,H° +98.9 kJ mol-1 A,H° = -95.4 kJ mol-1 A,Hº +108.3 kJ mol-1 (b) SO3(g) (c) N₂H4(g) (d) COC1₂(g) = SO₂(g) + 2 N₂(g) + 2 H₂(g) N₂(g) + 2 H₂(g) CO(g) + Cl₂(g) = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
To determine which of the given reactions would have an increase in the extent of the forward reacti...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions would you expect to have the larger rate at room temperature? Why? (Hint: Think of which would have the lower activation energy.) 2Ce4+(aq) + Hg22+(aq) 2Ce3+(aq) +...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
(a) In which of the following reactions would you expect the orientation factor to be least important in leading to reaction: NO + O NO2 or H + CI HCI? (b) How does the kinetic-molecular theory...
-
Transactions related to revenue and cash receipts completed by Acheville Architects Co. during the period September 2-30, 2014, are as follows: Sept. 2. Issued Invoice No. 793 to Nickle Co., $5,200....
-
What benefit is available to participants in a dividend reinvestment plan? How might the firm benefit?
-
Newell Co. uses special journals and a general journal. The following transactions occurred during May 2014. May 1 M. Newell invested $48,000 cash in the business in exchange for common stock. 2 Sold...
-
Assume that a RB Tire Store completed the following perpetual inventory transactions for a line of tires. Requirements 1. Compute cost of goods sold and gross profit under FIFO. 2. Compute cost of...
-
Gladmark Company produces two types of get-well cards: scented and regular. Drivers for the four activities are as follows: The following activity data have been collected: Inspecting products...
-
Your institution is experiencing a nursing shortage. As a result, quality of care has been impacted, the results of which have started to get out to the community. What approach would you take as the...
-
The following reaction represents the binding of oxygen by the protein hemoglobin (Hb): Explain how each of the following affects the amount of Hb:O 2 : (a) Increasing the temperature; (b) Decreasing...
-
What effect does increasing the volume of the system have on the equilibrium condition in each of the following reactions? (a) C(s) + HO(g) = CO(g) + H(g) (b) Ca(OH)2(s) + CO2(g) CaCO3(s) + HO(g) (c)...
-
What is a primary reason for projects to fail?
-
Everyone knows that Serial Killer Sam is guilty of a lot of stuff. If Bruce Banner goes home for the holidays and finds Serial Killer Sam camping out in his backyard in Illinois, how come he has to...
-
Locate a Law Society Tribunal decision from any Canadian jurisdiction that was determined between 2021 and 2023. The decision must be one that we have not studied in class. It need not be a...
-
Choose a company to make a recommendation to. This company can be any actual existing company, but should not be a fictional one. It must be real because you will need to research the company. Choose...
-
The following data have been extracted from the records of Puzzle Incorporated: Production level, in units Variable costs Fixed costs Mixed costs Total costs Required: a. Calculate the missing costs....
-
There are few areas as controversial as the use of deadly force by police officers who are attempting to apprehend a fleeing suspect. The current fleeing felon rule authorizes the police to use...
-
Multiple Choice Questions 1. Which of the following statements regarding the traditional manufacturing environment is not true? a. Factories were organized so that similar machines were grouped...
-
The Heese Restaurant Group manufactures the bags of frozen French fries used at its franchised restaurants. Last week, Heeses purchased and used 101,000 pounds of potatoes at a price of $ 0.70 per...
-
Give an example of how the capital expenditures budget affects other operating budgets
-
At the beginning of the period, the Fabricating Department budgeted direct labor of $22,500 and equipment depreciation of $7,000 for 900 hours of production. The department actually completed 750...
-
At the beginning of the period, the Assembly Department budgeted direct labor of $186,000 and property tax of $15,000 for 12,000 hours of production. The department actually completed 13,400 hours of...
-
Gallonte Inc. began operations in April of this year. It makes all sales on account, subject to the following collection pattern: 20% are collected in the month of sale; 50% are collected in the...
-
A dairy company processed raw milk for $62,000. This raw milk can be converted into the following types of milk with listed sales values. Joint Products Whole milk 2% milk Skim milk Sales Value Total...
-
Assume that in October 2022 the Schmidt Machinery Company ( Exhibit 14.1 ) manufactured and sold 970 units for $837 each. During this month, the company incurred $499,550 total variable costs and...

Study smarter with the SolutionInn App


