In 1860, Stanislao Cannizzaro showed how Avogadros hypothesis could be used to establish the atomic masses of
Question:
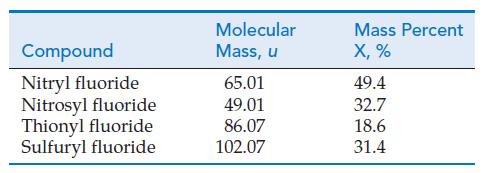
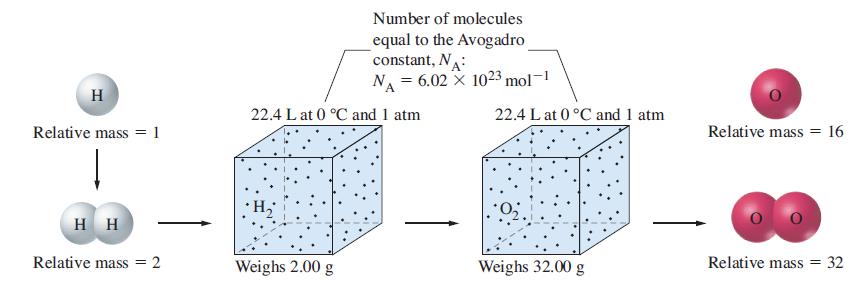
In 1860, Stanislao Cannizzaro showed how Avogadro’s hypothesis could be used to establish the atomic masses of elements in gaseous compounds. Cannizzaro took the atomic mass of hydrogen to be exactly one and assumed that hydrogen exists as H2 molecules (molecular mass = 2). Next, he determined the volume of H2(g) at 0.00 °C and 1.00 atm that has a mass of exactly 2 g. This volume is 22.4 L. Then he assumed that 22.4 L of any other gas would have the same number of molecules as in 22.4 L of H2(g). (Here is where Avogadro’s hypothesis entered in.) Finally, he reasoned that the ratio of the mass of 22.4 L of any other gas to the mass of 22.4 L of H2(g) should be the same as the ratio of their molecular masses. The sketch below illustrates Cannizzaro’s reasoning in establishing the atomic weight of oxygen as 16. The gases in the table all contain the element X. Their molecular masses were determined by Cannizzaro’s method. Use the percent composition data to deduce the atomic mass of X, the number of atoms of X in each of the gas molecules, and the identity of X.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





