In a capillary rise experiment, the height (h) to which a liquid rises depends on the density
Question:
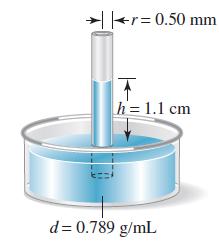
In a capillary rise experiment, the height (h) to which a liquid rises depends on the density (d) and surface tension (ɣ) of the liquid and the radius of the capillary (r). The equation relating these quantities and the acceleration due to gravity (g) is h = 2ɣ/dgr. The sketch provides data obtained with ethanol. What is the surface tension of ethanol?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





