The BornFajansHaber cycle uses thermodynamic cycles to determine lattice energy. An alternative to the BornFajansHaber method is
Question:
The Born–Fajans–Haber cycle uses thermodynamic cycles to determine lattice energy. An alternative to the Born–Fajans–Haber method is one based on fundamental principles. Because the dominant interactions in an ionic crystal are Coulomb interactions, we can use the theory of electrostatics to calculate the lattice energy. Kapustinskii used these ideas and proposed the following equation:
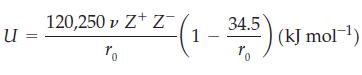
where the number of ions per formula unit is given by ν and r0 is equal to the sum of the ionic radii, r+ + r- (pm) . Use the equation to complete the following table:

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





