In a student experiment to confirm Hesss law, the reaction was carried out in two different ways.
Question:
In a student experiment to confirm Hess’s law, the reaction
![]()
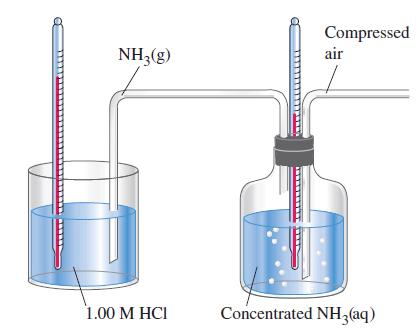
was carried out in two different ways. First, 8.00 mL of concentrated NH3(aq) was added to 100.0 mL of 1.00 M HCl in a calorimeter. (The NH3(aq) was slightly in excess.) The reactants were initially at 23.8 °C, and the final temperature after neutralization was 35.8 °C. In the second experiment, air was bubbled through 100.0 mL of concentrated NH3(aq), sweeping out NH3(g) (see sketch). The NH3(g) was neutralized in 100.0 mL of 1.00 M HCl. The temperature of the concentrated NH3(aq) fell from 19.3 to 13.2 °C. At the same time, the temperature of the 1.00 M HCl rose from 23.8 to 42.9 °C as it was neutralized by NH3(g). Assume that all solutions have densities of 1.00 g/mL and specific heat capacities of 4.18 J g-1 °C-1.
(a) Write the two equations and ¢rH values for the processes occurring in the second experiment. Show that the sum of these two equations is the same as the equation for the reaction in the first experiment.
(b) Show that, within the limits of experimental error, ΔrH for the overall reaction is the same in the two experiments, thereby confirming Hess’s law.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





