Refer to Example 7-5 dealing with the work done by 0.100 mol He at 298 K in
Question:
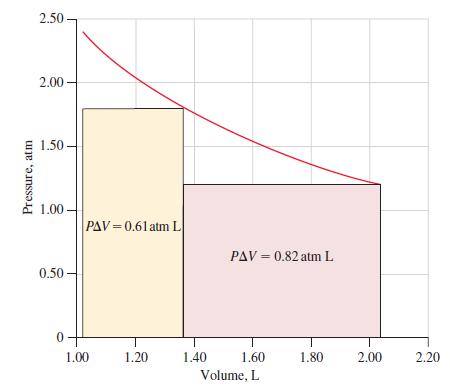
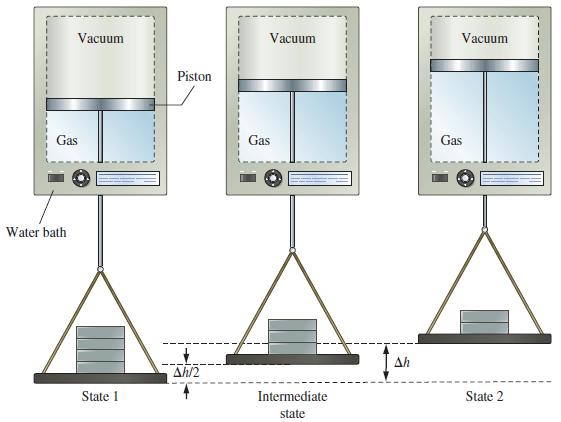
Refer to Example 7-5 dealing with the work done by 0.100 mol He at 298 K in expanding in a single step from 2.40 to 1.20 atm. Review also the two-step expansion (2.40 atm → 1.80 atm → 1.20 atm) described on page 261 (see Figure 7-11).
(a) Determine the total work that would be done if the He expanded in a series of steps, at 0.10 atm intervals, from 2.40 to 1.20 atm.
(b) Represent this total work on the graph below, in which the quantity of work done in the two-step expansion is represented by the sum of the colored rectangles.
(c) Show that the maximum amount of work would occur if the expansion occurred in an infinite number of steps. To do this, express each infinitesimal quantity of work as dw = -P dV and use the methods of integral calculus (integration) to sum these quantities. Assume ideal behavior for the gas.
(d) Imagine reversing the process, that is, compressing the He from 1.20 to 2.40 atm. What are the maximum and minimum amounts of work required to produce this compression? Explain.
(e) In the isothermal compression described in part (d), what is the change in internal energy assuming ideal gas behavior? What is the value of q?
(f) Using the formula for the work derived in part (c), obtain an expression for q/T. Is this new function a state function? Explain.
Example 7-5
Suppose the gas in Figure 7-8 is 0.100 mol He at 298 K, the two weights correspond to an external pressure of 2.40 atm in Figure 7-8(a), and the single weight in Figure 7-8(b) corresponds to an external pressure of 1.20 atm. How much work, in joules, is associated with the gas expansion at constant temperature?
Figure 7-8
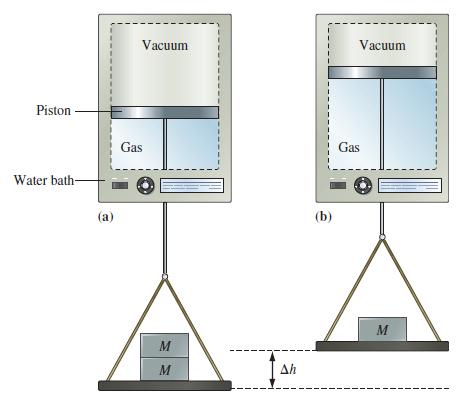
Figure 7-11
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




