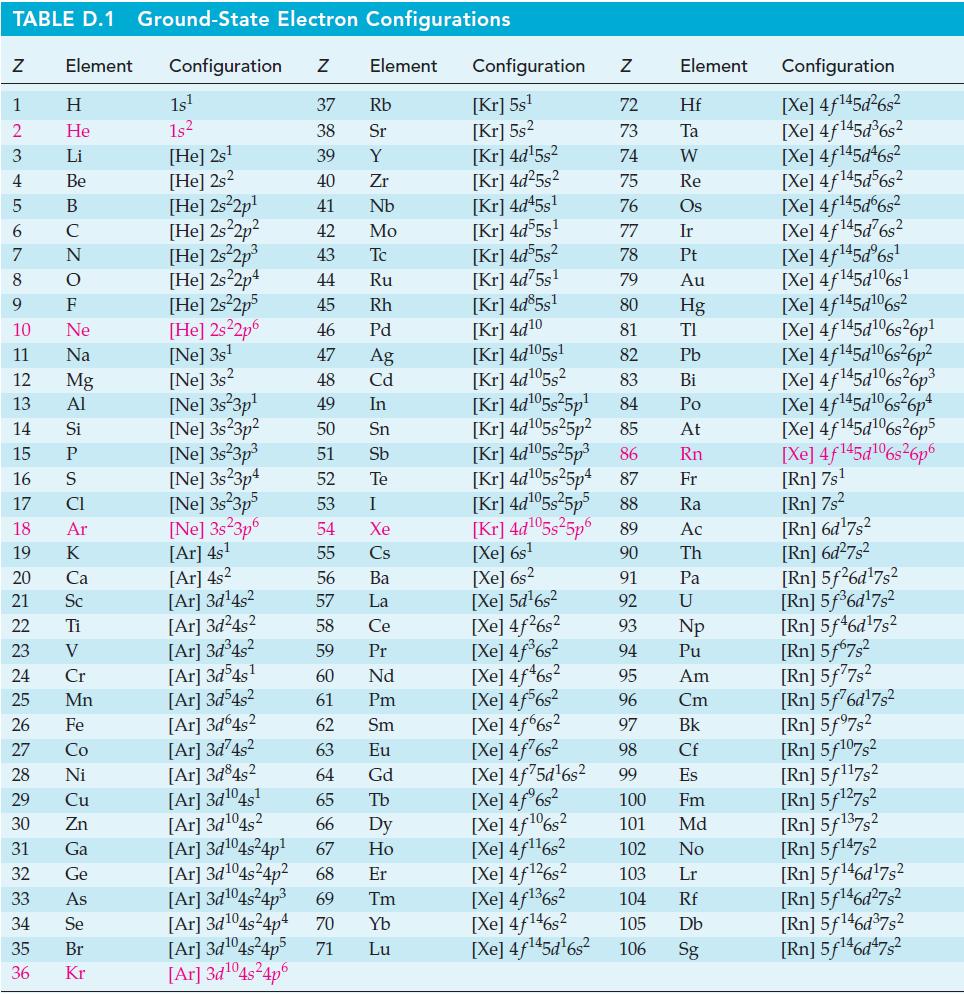
In Example 13-2, we were unable to conclude by inspection whether r S for the reaction
Question:
In Example 13-2, we were unable to conclude by inspection whether ΔrS° for the reaction CO(g) + H2O(g) → CO2(g) + H2(g) should be positive or negative. Use data from Appendix D to obtain ΔrS° at 298 K.
Example 13-2
Predict whether each of the following processes involves an increase or a decrease in entropy or whether the outcome is uncertain.
(a) The decomposition of ammonium nitrate (a fertilizer and a highly explosive compound):
![]()
(b) The conversion of SO2 to SO3 (a key step in the manufacture of sulfuric acid):
![]()
(c) The extraction of sucrose from cane sugar juice:
![]()
(d) The “water gas shift” reaction (involved in the gasification of coal):
![]()
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





