One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules,
Question:
One glucose molecule, C6H12O6(s), is converted to two lactic acid molecules, CH3CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the standard enthalpy for glycolysis.
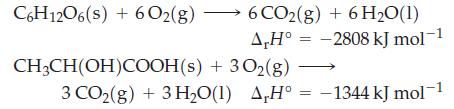
Transcribed Image Text:
C6H12O6(s) + 602(g) 6 CO2(g) + 6H₂O(1) A,H° ΔΗ° CH₂CH(OH)COOH(s) + 3O₂(g) 3CO2(g) +3H,O(1) AH° -2808 kJ mol-1 -1344 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the standard enthalpy for glycolysis using the given information we can use Hesss Law H...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In biological cells that have a plentiful supply of oxygen, glucose is oxidized completely to CO 2 and H 2 O by a process called aerobic oxidation. Muscle cells may be deprived of O 2 during vigorous...
-
Choose a conflict situation you experienced in a work setting. Make sure that your illustration is work-related and not personal. Prepare a formal report, with recommendations you would make, based...
-
Can we use servomotor for position control? Support the answer with necessary details
-
Consider the hydrogen atom, and assume that the proton, instead of being a point- source of the Coulomb field, is uniformly charged sphere of radius R ( < < ao), so that the Coulomb potential is now...
-
Assume that you must make a presentation to the marketing staff explaining the difference between product and period costs. Your supervisor tells you the marketing staff would also like clarification...
-
Pet Corporation owns 100 percent (300,000 shares) of the outstanding shares of Sap Corporations common stock on January 1, 2011. Its Investment in Sap account on this date is $4,400,000, equal to...
-
For a sample of size n = 15, the following values were obtained: b0 = 3.71, b1 = 8.38, se = 1.13, (x x)2 = 7.71, x = 13.16. Construct a 95% prediction interval for an individual response when x = 8.
-
PrimeTime Sportswear is a custom imprinter that began operations six months ago. Sales have exceeded management's most optimistic projections. Sales are made on account and collected as follows: 60%...
-
Forecast Income Statement and Balance Sheet Following are the income statement and balance sheet for Medtronic PLC. Note: Complete the entire question using the following Excel template: Excel...
-
Implied Volatility. Replicate the Implied Volatility Smile Figure on Page 12 of LN3, using current Call options data on the S&P500 (SPX) maturing on January 20, 2023. Please state the assumptions you...
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard heats of combustion ( r H) of buta-1,3-diene, C 4 H 6 (g); butane, C 4 H 10 (g); and H 2 (g) are -2540.2, -2877.6, and -285.8 kJ mol-1, respectively. Use these data to calculate the heat...
-
A 4-m-long beam is subjected to a variety of loadings. (a) Replace each loading with an equivalent force-couple system at end A of the beam. (b) Which of the loadings are equivalent? 400 N 200 N-m A...
-
Babe Ruth was paid $80,000 in 1931. When it was pointed out that this was morethan President Hoover made, he replied, I had a better year than he did. Using a base 100 in 1967, the CPI was 45.6 in...
-
Make a List of typical items paid for through borrowing. Share your list with friends and relatives and ask them to quickly categorize the debts as good or bad. Record their reactions. Then explain...
-
According to this chapter, "planning and budgeting require control." Talk to several friends, family, or acquaintances about the strategies they routinely or occasionally use to control spending and...
-
On January 11, 2008, the value of the Dow Jones Industrial Average was 12,606.30, and the divisor was 0.123017848. Explain why you either agree or disagree with these statements: a. The average price...
-
"The more you make, the more necessities there are." Discuss this quote with friends or relatives representing different stages of the life cycle and income levels. Can they recall specific goods or...
-
Discuss each of the following as they are related to assessing bond market behavior. a.Bond yields b.Bond indexes
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
Distinguish between counterbalancing and non-counterbalancing errors. Give an example of each.
-
Discuss and illustrate how a correction of an error in previously issued financial statements should be handled.
-
Prior to 2010, Heberling Inc. excluded manufacturing overhead costs from work in process and finished goods inventory. These costs have been expensed as incurred. In 2010, the company decided to...
-
On January 1, 2024, Madison Products issued $40 million of 6%, 10-year convertible bonds at a net price of $40.8 million. Madison recently issued similar, but nonconvertible, bonds at 99 (that is,...
-
On January 1st, 2021, Element Fire Doors purchased an additional welding machinery. The machinery cost $150,000 and has an estimated residual value of $24,000. The machinery is expected to be used...
-
Quary Company is considering an investment in machinery with the following information. The company's required rate of return is 15%. Initial investment Useful life Salvage value $ 204,000 Materials,...

Study smarter with the SolutionInn App


