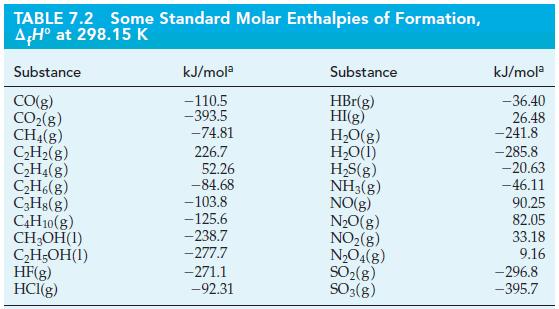
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of
Question:
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions.
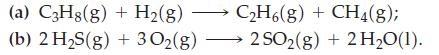
Table 7.2
Eq. 7.22
![A,H [cx AHc + dx AHD + ...]-[ax AHA + bx AHB +...] (7.22) weighted sum of A H values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/241654de711110c61699604236540.jpg)
Transcribed Image Text:
(a) C3H8(g) + H₂(g) → C₂H6(g) + CH4(g); (b) 2 H₂S(g) + 30₂(g) →→→2SO₂(g) + 2 H₂O(1).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a AHTxnAH products AH reactants ...View the full answer

Answered By

Nandana Wijayarathna
I am a highly experienced writer in several areas,
Business management
Information technology
Business administration
Literature
Biology
Environmental science
History
4.50+
161+ Reviews
399+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard enthalpy of reaction in the following reaction. Tables 7.2 Tables 7.3 Eq. 7.22 NH4+ (aq) +...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use standard enthalpies of formation from Table 7.2 to determine r H at 25 C for the following reaction. Table 7.2 2 Cl(g) + 2 HO(1) 4 HCl(g) + O(g) A,H = ?
-
Riffa Football Club is planning to organize a football tournament to raise charity funds. The estimated costs per match Amount paid to Players, Coaches and Referees BHD 1,400; Ground Rent BHD 250;...
-
Refer to Decision Maker, Purchase Manager, in this chapter. Assume that you are the motorcycle manufacturers managerial accountant. The purchasing manager asks you about preparing an estimate of the...
-
Find the change in length of the bar loaded as shown in Fig. 13.48. Take \(E=200 \mathrm{GPa}\). 50 kN 20 mm 15 mm 0.2 m 0.3 m Fig. 13.48 20 mm 50 kN 0.2 m
-
What is the relationship between a Problem Space and an Opportunity Space?
-
The July 1, 20X5, trial balance for the Bond Redemption and Interest Debt Service Fund of the County of Hawaii, Hawaii, is presented here. The resources of the fund are committed to debt service. The...
-
Consider the four characteristics of conscious capitalism: conscious leadership, stakeholder orientation, conscious culture, and higher purpose. Describe how these characteristics together can...
-
How could the city have avoided the outcome? Explain. Do you think that it would have made sense for the city to consider the particulars of the circumstances here, such as that these were...
-
The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol. C6H12O6(s)- 2 CH3CHOH(1) + 2...
-
One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules, CH 3 CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the...
-
Skim through Appendix A on Microsoft Project 2016 (available on the Companion website for this text). Review information about Project 2016 from the Microsoft website (www.microsoft.com). Research...
-
The responses to which questions lead to determining decision context? Select answer from the options below How fast and what format is the information needed? Who will use it, and why is the...
-
This scenario-based assessment is in three parts: Provide a cited overview of issues related to diversity of a workforce. Analyze and provide recommendations for a new policy for in and out-of-office...
-
They are planning to take on Canada again - they will have to play better. What do we call this type of growth?
-
How do you tend to think about percent? Do you break down the word: per cent to per hundred? Do you think of it as a ratio: 1/100? Do you think of it as a decimal? Do you have any shortcuts for...
-
Ritchie Manufacturing Company makes a product that it sells for $140 per unit. The company incurs variable manufacturing costs of $73 per unit. Variable selling expenses are $11 per unit, annual...
-
A manufacturing company produces plastic pipes that are specified to be 10 inches long and 1/8 inch thick with an opening of 3/4 inch. These pipes are molded on two different machines. To maintain...
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a seven-year operating lease agreement. The contract calls for quarterly rent payments of $25,000 each....
-
Discuss how a change to the LIFO method of inventory valuation is handled when it is impracticable to determine previous LIFO inventory amounts.
-
How should consolidated financial statements be reported this year when statements of individual companies were presented last year?
-
Simms Corp. controlled four domestic subsidiaries and one foreign subsidiary. Prior to the current year, Simms Corp. had excluded the foreign subsidiary from consolidation. During the current year,...
-
Questions Q3(a) and Q3(b) are based on Figure Q3. Target's Supermarket, a small chain of grocery stores in Parit Raja, is building a website to allow customers to place orders for groceries and other...
-
Given the template of a class named ChickenEgg as follows: public class ChickenEgg { private char grade; //A, B, C private int numEgg; //number of eggs //Methods: //constructors, mutators, accessors,...
-
Questions Q2(a)-Q2(c) are based on Figure Q2 Tuah enterprise produces parts for the automobile industry. The executive board has decided that the firm's product catalog shall be available on the web...

Study smarter with the SolutionInn App


