Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression
Question:
Show that in terms of mole fractions of gases and total gas pressure the equilibrium constant expression for
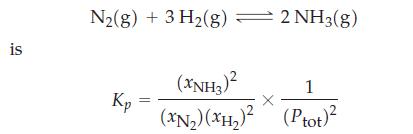
Transcribed Image Text:
is N₂(g) + 3 H₂(g) = 2 NH3(g) Kp (XNH3)² 1 (XN₂) (XH₂)² (Ptot)²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The equilibrium constant expression for a gasphase reaction can be written in terms of either partia...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Methane (CH 4 ) gas flows into a combustion chamber at a rate of 200. L/ min at 1.50 atm and ambient temperature. Air is added to the chamber at 1.00 atm and the same temperature, and the gases are...
-
A continuous distillation unit, consisting of a perforated-tray column together with a partial reboiler and a total condenser, is to be designed to operate at atmospheric pressure to separate ethanol...
-
Write the equilibrium constant expression for the dissolution of ammonia in water: Use this equilibrium constant expression to estimate the partial pressure of NH 3 (g) over a solution containing 5 x...
-
Olmsted Co. has small computer chips assembled in Poland and transports the final assembled products to the parent, where they are sold by the parent in the U.S. The assembled products are invoiced...
-
Columbia Paper has the following stockholders equity account. The firms common stock has a current market price of $30 per share. Preferred stock .............$100,000 Common stock (10,000 shares at...
-
Visit some Web sites and identify all of the controls used for navigation and input. Are they all obvious? Discuss some differences in visibility and affordance of the controls.
-
Crestview Pool Supply has 10,000 shares of \(\$ 1\) par common stock outstanding. Crestview distributes a \(10 \%\) stock dividend when the market value of its stock is \(\$ 15\) per share. 1....
-
Bartlet Financial Services Company holds a large portfolio of debt and share securities as an investment. The total fair value of the portfolio at December 31, 2017, is greater than total cost. Some...
-
5. How would strategic planning for human resources differ if Scerba were hired by a sports organization that did not have a temporary workforce? (4 pts)
-
A mixture of H 2 S(g) and CH 4 (g) in the mole ratio 2 : 1 was brought to equilibrium at 700 C and a total pressure of 1 atm. On analysis, the equilibrium mixture was found to contain 9.54 x 10 -3...
-
What is the apparent molar mass of the gaseous mixture that results when COCl 2 (g) is allowed to dissociate at 395 C and a total pressure of 3.00 atm? Think of the apparent molar mass as the molar...
-
Chevelle, Inc., is obligated to pay its creditors $8,400 during the year. a. What is the value of the shareholders' equity if assets equal $9,300? b. What if assets equal $6,900?
-
A block of solid copper sits on a flat, level shelf. Copper has a density of 8.94 x 103 kg/m. The mass of the block is 25.0 kg. (a) What is the volume of the block (in m)? m (b) The amount of surface...
-
1) Evaluate the speed of light in a material with an index of refraction n = 1.33, round your answer to the nearest whole number. 2) A laser is pointed at a mysterious material as seen in the diagram...
-
You have to deliver some 5.0-kg packages from your home to two locations. You drive for 2.0 h at 30 mi/h due east (call this segment 1 of your trip), then turn around and drive due west for 30 min at...
-
You are an expatriate (with a spouse and a 6-year-old daughter) sent on assignment for one year to Hong Kong. What should you do to prepare for departure? Why? What training should you or your family...
-
A soap bubble (n = 1.28) having a wall thickness of 127 nm is floating in air. (a) What is the wavelength of the visible light that is most strongly reflected? Your response differs from the correct...
-
B & B Manufacturing Co. was organized on January 1 of the current year. Outside investors who financed the business stipulated that the company must show a profit by the sixth month or the financing...
-
Les has collected stamps in his spare time for years. He purchased many of his stamps at a price much lower than the current market value. Les recently lost his job as a carpenter. Since his wife...
-
Washington Co. operates a chain of bookstores. The company maintains a defined contribution pension plan for its employees. The plan requires quarterly installments to be paid to the funding agent,...
-
In a recent years financial statements, Procter & Gamble showed an unfunded pension liability of $2,637 million and a periodic pension cost of $183 million. Explain the meaning of the $2,637 million...
-
Lachgar Industries warrants its products for one year. The estimated product warranty is 4% of sales. Assume that sales were $210,000 for June. In July, a customer received warranty repairs requiring...
-
Write a Java class called Iso Triangle that reads from the user an integer (h) represents the length of the hypotenuse of an isosceles right triangle. Then calculates and prints the area of this...
-
The following are historical demand data: ACTUAL YEAR 2011 SEASON DEMAND Spring 205 Summer 151 Fall 379 Winter 560 2012 Spring 481 Summer 268 Fall Winter 679 959 Use regression analysis on...
-
Suppose you have a 1-year old son and you want to provide $200,000 in 17 years towards his college education. You currently have $25,000 to invest. What interest rate must you earn to have the...

Study smarter with the SolutionInn App


