Starting with 0.280 mol SbCl 3 and 0.160 mol Cl 2 , how many moles of SbCl
Question:
Starting with 0.280 mol SbCl3 and 0.160 mol Cl2, how many moles of SbCl5, SbCl3, and Cl2 are present when equilibrium is established at 248 °C in a 2.50 L flask?
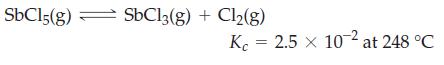
Transcribed Image Text:
SbC15(g) = SbCl3(g) + Cl₂(g) Kc 2.5 x 10-2 at 248 °C =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To solve this problem we can use the concept of the reaction quotient Q and the equilibrium constant ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) If 0.150 mol H 2 (g) and 0.200 mol I 2 (g) are introduced into a 15.0 L flask at 445 C and allowed to come to equilibrium, how many moles of HI(g) will be present? (B) Suppose the equilibrium...
-
In the contact process, sulfuric acid is manufactured by first oxidizing SO2 to SO3, which is then reacted with water. The reaction of SO2 with O2 is A 2.000-L flask was filled with 0.0400 mol SO2...
-
When an acid reacts with a base: 1) This is a neutralization reaction 2) Pink color will appear in the resulting solution 3) Both of the answers are correct 4) None of the answers is correct QUESTION...
-
For light that originates within a liquid and strikes the liquid-air interface, the critical angle is 39. What is Brewster's angle for this light?
-
Paul Scott has a 2008 Cadillac that he wants to update with a geo-tracker device so he will have access to road maps and directions. After-market equipment can be fitted for a flat fee of $500, and...
-
Iverson owned Iverson Motor Company, an enterprise engaged in the repair as well as the sale of Oldsmobile, Rambler, and International Harvester Scout automobiles. Forty percent of the businesss...
-
When the plaintiff was 16, he was employed by Kmart as a cashier. At the end of his training, he was required to read Kmarts policy agreement, which included an agreement to submit all employment...
-
The trial balance of Avtar Sandhu Co. shown below does not balance. Each of the listed accounts has a normal balance per the general ledger. An examination of the ledger and journal reveals the...
-
A small electric immersion heater is used to heat 87 g of water for a cup of instant coffee. The heater is labeled "120 watts" (it converts electrical energy to thermal energy at this rate)....
-
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl 2 (g) in a 3.050 L flask at 668 K, how many moles of Cl 2 (g) will be present at equilibrium? CO(g) + Cl2(g) = COC1(g) Kc = 1.2 x 10 at 668 K
-
In the reaction CO(g) + H 2 O(g) CO 2 (g) + H 2 (g), K c = 31.4 at 588 K. Equal masses of each reactant and product are brought together in a reaction vessel at 588 K. (a) Can this mixture be at...
-
Describe the differences in the planned audit approaches for Clients A and B and the reasons for such differences. Discuss.
-
Cylinder in magnetic fields (8pts) A uniform magnetic field of magnitude Bo points in the positive z- direction. An infinitely long solid cylinder runs parallel to and is centered on the z-axis. The...
-
Discuss how does ARP spoofing work? What is port mirroring and why is it used?
-
A project requires an initial investment of $2,400,000 depreciated straight-line to $0 in 10 years. The investment is expected to generate annual sales of $700,000 with annual costs of $450,000 for...
-
n preparation for Thanksgiving Day, the Save-You-More Store has stacked cans of cherry pie filling in a triangular pyramid. The top of the pyramid has a single can, the second row has three cans, and...
-
Discuss the application of the Divide-and-Conquer approach in computer science?
-
The inventory at June 1 and costs charged to Work in Process-Department 60 during June are as follows: 3800 units, 80% completed ............ $ 60,400 Direct materials, 32,000 units ..............
-
From 1970 to 1990, Sri Lanka's population grew by approximately 2.2 million persons every five years. The population in 1970 was 12.2 million people.What is the best formula for P, Sri Lanka's...
-
The data related to Acclaim Sporting Goods Company's factory overhead cost for the production of 50,000 units of product are as follows: Productive capacity at 100% of normal was 75,000 hours, and...
-
Scientific Molded Products Inc. prepared the following factory overhead cost budget for the Trim Department for August 2010, during which it expected to use 10,000 hours for production: Scientific...
-
Orion Manufacturing Company incorporates standards in its accounts and identifies variances at the time the manufacturing costs are incurred. Journalize the entries to record the following...
-
Identify the online video and briefly explain the topic, and provide some insight into the speaker. Provide a minimum three-paragraph summary and analysis of the connection between the online video...
-
Sam and his wife become first - time parents. His manager was going to promote him to Assistant Manager but since there would be travel involved, the manager decided to promote another employee who...
-
Drafting about three hundred words or so for each question using the sources below and (any additional if you find them more relevant) to the question. How can decision-makers anticipate, prevent,...

Study smarter with the SolutionInn App


