The bond angle in the H 2 O molecule is given as 104 and the resultant dipole
Question:
The bond angle in the H2O molecule is given as 104° and the resultant dipole moment as μ = 1.84 D.
(a) By an appropriate geometric calculation, determine the value of the H—O bond dipole in H2O.
(b) Use the same method as in part (a) to estimate the bond angle in H2S, given that the H—S bond dipole is 0.67 D and that the resultant dipole moment is μ = 0.93 D.
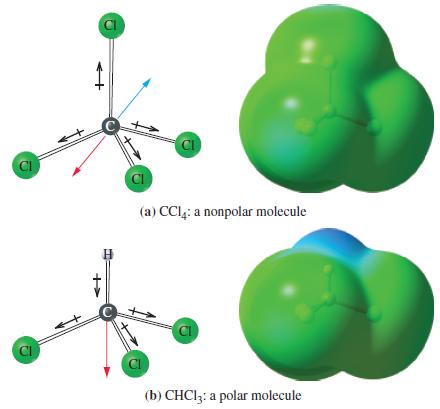
(c) Refer to Figure 10-16. Given the bond dipoles 1.87 D for the C—Cl bond and 0.30 D for the C—H bond, together with μ = 1.04 D, estimate the H—C—Cl bond angle in CHCl3.
Figure 10-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





