Use data from Table 7.2, together with the fact that r H = -3509 kJ mol
Question:
Use data from Table 7.2, together with the fact that ΔrH° = -3509 kJ mol-1 for the complete combustion of pentane, C5H12(l), to calculate ΔrH° for the reaction below.
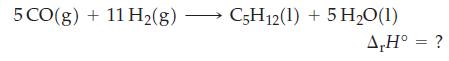
Table 7.2
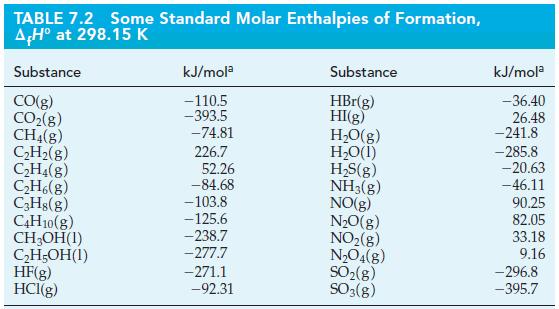
Transcribed Image Text:
5 CO(g) + 11 H₂(g) - C5H12(1) + 5 H₂O(1) A,H° = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the standard enthalpy change AH for the ...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Table to estimate ÎH for the combustion of methane (CH4), as shown below: Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122...
-
Create an accompanying report that draws on relevant theories and concepts within Block 1 to explain and justify the decisions made in producing your team's piece of marketing communication. Context...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The symmetrical three-phase load is A-connected, with Z-(4+ j3) 22 and the phase voltage 220 V, find IL, IP, UL and P of the three loads. 7.(10 score) The symmetrical three-phase load is Y-connected....
-
Assume that you must make a presentation to a client explaining the difference between prime and conversion costs. The client makes and sells 200,000 cookies per week. The client tells you that her...
-
Assume that Wal-Mart decided to acquire major retailing rival Target Corporation on January 31, 2010. Targets fiscal year ended on January 30, 2010, but you may assume that the year ends are...
-
Use the data in Exercise 19 in Section 13.1 for the following: a. Compute a point estimate for the mean number of calories in fast-food products that contain 15 grams of protein. b. Construct a 95%...
-
Ogwood Companys Johnstown Division is a small manufacturer of wooden household items. Al Rivkin, division controller, plans to implement a standard-costing system. Rivkin has collected information...
-
What are the principal areas that need to be addressed to strengthen the right-hand side of its balance sheet?
-
You are an Examiner for the Refund Integrity Program in the GST/HST Audit Division of the Canada Revenue Agency (CRA). Using sophisticated analytical tools, you are able to identify GST/HST returns...
-
Use data from Table 7.2 and r H for the following reaction to determine the standard enthalpy of formation of CCl 4 (g) at 25 C and 1 bar. Table 7.2 CH4(g) + 4Cl(g) CCl4(g) + 4HCI(g) AH -397.3 kJ mol
-
Use data from Table 7.2 to determine the standard heat of combustion of C 2 H 5 OH(l), if reactants and products are maintained at 25 C and 1 bar. Table 7.2 TABLE 7.2 AH at 298.15 K Substance CO(g)...
-
In each case, compute the characteristic polynomial cT(x). (a) T : R2 R2, T(a, b) = (a - b, 2b - a) (b) T: R2 R2, T(a, b) = (3a + 5b, 2a + 3b) (c) T: P2 P2, T(a + bx + cx2) = (a - 2c) + (2a + b +...
-
Your friend recently completed a home addition that included a new laundry room and larger master bath. There appears to be a problem with the plumbing drain. If the master bath commode is flushed...
-
Explain why Social Security benefits are more important for lower income households than for higher income households.
-
Name the two securities regulation organizations. What is their primary purpose for securities market regulation?
-
Make a list of criteria that you would use in selecting the executor of your estate and the heirs named to inherit your assets. How would these criteria change if you were selecting a guardian for...
-
Define the following health care policy terms: guaranteed renewable and exclusions. How has the Affordable Care Act changed the coverage for preexisting conditions and mental disorders?
-
What benefits does an investment club offer the small investor? Would you prefer to join a regular or an online club, and why?
-
Interview managers at three companies in your area about their use of ERP. How have their experiences been similar? What accounts for the similarities and differences?
-
Classification of Costs and Interest Capitalization On January 1, 2010, Blair Corporation purchased for $500,000 a tract of land (site number 101) with a building. Blair paid a real estate brokers...
-
Interest during Construction Grieg Landscaping began construction of a new plant on December 1, 2010. On this date the company purchased a parcel of land for $139,000 in cash. In addition, it paid...
-
Capitalization of Interest Laserwords Inc. is a book distributor that had been operating in its original facility since 1985. The increase in certification programs and continuing education...
-
On December 1, 2020, Tammy Company made a basket purchase of land, a building, and equipment for $320,000. Appraised values at the time of the purchase were: land $150,000; building, $200,000; and...
-
WeDeliver Incorporated is the world's leading express-distribution company. In addition to its 643 aircraft, the company has more than 57,000 ground vehicles that pick up and deliver packages. Assume...
-
One aspect of the module focused on Okun's Law, which describes the relationship between Real GDP growth and the change in the unemployment rate and may be approximated as follows: Real GDP Growth =...

Study smarter with the SolutionInn App


