When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm 3 )
Question:
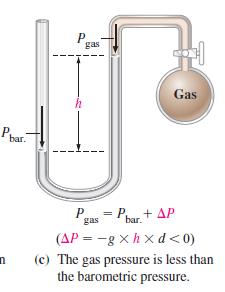
When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm3) the barometric pressure is 748.2 mmHg, and the difference in mercury levels is 8.6 mmHg. What is the gas pressure Pgas?
Figure 6-5(c)
Transcribed Image Text:
Ph bar. n P h gas P gas 220 = Pbar + AP Gas (AP=-gxhxd<0) (c) The gas pressure is less than the barometric pressure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Analyze We must first establish which is greater the barometric pressure or the ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Suppose that the mercury level in Example 6-2 is 7.8 mm higher in the arm open to the atmosphere than in the closed arm. What would be the value of P gas ? (B) Suppose P bar. and P gas are those...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A cylindrical glass beaker of height 1.520 m rests on a table. The bottom half of the beaker is filled with a gas, and the top half is filled with liquid mercury that is exposed to the atmosphere....
-
Briefly explain the differences between copyrights and patents.
-
Refer to the data and analysis developed for the Magee Company in Exhibits 8.5 and 8.6. Evaluate the alternatives using a cost of capital of 12 percent.
-
An object of irregular shape has a characteristic length of \(L=0.5 \mathrm{~m}\) and is maintained at a uniform surface temperature of \(T_{s}=400 \mathrm{~K}\). When placed in atmospheric air at a...
-
What are the business benefits of an EA? Explain why it is necessary to ensure that an EA maintains alignment between IT and business strategy?
-
The following data are for the A, B, and C Companies: Required a. Compute the Z score for each company. b. According to the Altman model, which of these firms is most likely to experience...
-
Consider the situation of John, aged 15, who is found to be a mature minor. He decides to stop cancer treatment. His oncology team supports his decision. He has been in treatment for two years and...
-
Calculate the height of a mercury column required to produce a pressure (a) Of 0.984 atm; (b) Of 928 Torr; (c) Equal to that of a column of water 142 ft high.
-
Explain how the action of a water siphon is related to that of a suction pump.
-
The comparative balance sheet of Winner's Edge Sporting Goods, Inc., for December 31, 2007 and 2006, is as follows: The income statement for the year ended December 31, 2007, is as follows: The...
-
Define a relation R on Z as follows: m, n Z, m Rn 5 (m - n). Which one of the following choices is not true? (-8) R 11 01R (-9) 2 R 13 5 R 5
-
Establish how many times statement x = 2* x is executed in the asymptotic sense: j = n; x = 1; while (j >= 1){ for (i = 1; i
-
P3) Given C++ code, answer the following scope related questions: int x = 5; int y = 10; { int y = 20; { y = y + 10; X = x + 10; } cout
-
What sequence of commands we used to download and execute 37292.c privilege escalation exploit on victiml? Hint: choices with extra command lines are wrong a. $>cd tmp $>wget $>gcc 37292.c $>./a.out...
-
4. Give the following arrays int a[5] { 1,5,3,4,2); char c[6]={'a','r','r','a', 'y', 's'}; string d[3]={"try", "enjoy", "programming"}; we want to display all arrays by overloading the Display...
-
Cotton White, Inc., makes specialty clothing for chefs. The company reported the following costs for 2012: Factory rent ............ $42,000 Company advertising ......... 18,000 Wages paid to...
-
Willingness to pay as a measure of a person's value for a particular good measures the maximum a person would be willing to pay requires that payment actually be made depends on the satisfaction that...
-
Why didnt the FASB cover both types of postretirement benefitspensions and healthcarein the earlier pension accounting rules?
-
What are the major differences between postretirement healthcare benefits and pension benefits?
-
What is the difference between the APBO and the EPBO? What are the components of postretirement expense?
-
1. Explain Amazon Inc free cash flow history. 2. What is normalization adjustment ? 3. What are three examples of balance sheet normalization adjustments that might appear on Amazon's balance sheet...
-
Business success is often tied to effectively managed strategies. Using the Internet, study Starbuck's current performance. Based on analysis, do you judge Starbucks to be a success? Why or why not?...
-
Go to SEC website of Amazon that has leases and review their (consolidated) financial statements.Here is one of the example link...

Study smarter with the SolutionInn App


