You want to test the following proposed mechanism for the oxidation of HBr. You find that the
Question:
You want to test the following proposed mechanism for the oxidation of HBr.
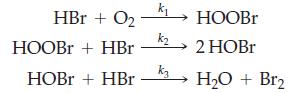
You find that the rate is first order with respect to HBr and to O2. You cannot detect HOBr among the products.
(a) If the proposed mechanism is correct, which must be the rate-determining step?
(b) Can you prove the mechanism from these observations?
(c) Can you disprove the mechanism from these observations?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





