During absorption of low-solubility gases, mass transfer from a highly concentrated gas mixture to a very dilute
Question:
During absorption of low-solubility gases, mass transfer from a highly concentrated gas mixture to a very dilute liquid solution frequently takes place. In that case, although it is appropriate to use a $k$-type mass-transfer coefficient in the liquid phase, an $F$-type coefficient must be used in the gas phase. Since dilute liquid solutions usually obey Henry's law, the interfacial concentrations during absorption of low-solubility gases are related through $y_{A, i}=m x_{A, i}$.
(a) Show that, under the conditions described above, the gas interfacial concentration satisfies the equation
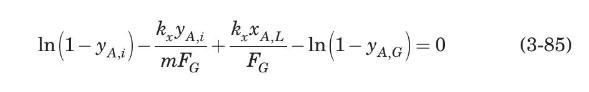
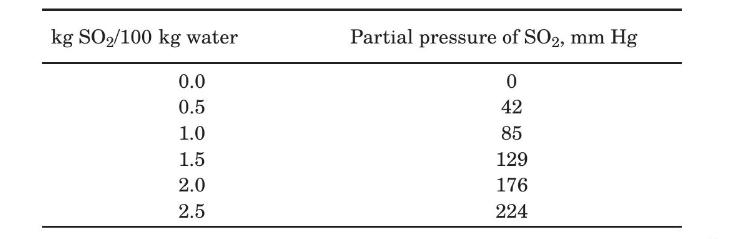
(b) In a certain apparatus used for the absorption of $\mathrm{SO}_{2}$ from air by means of water, at one point in the equipment the gas contained $30 \% \mathrm{SO}_{2}$ by volume and was in contact with a liquid containing $0.2 \% \mathrm{SO}_{2}$ by mol. The temperature was $303 \mathrm{~K}$ and the total pressure $1 \mathrm{~atm}$. Estimate the interfacial concentrations and the local $\mathrm{SO}_{2}$ molar flux. The mass-transfer coefficients were calculated as $F_{G}=2.0 \mathrm{~mol} /$ $\mathrm{m}^{2} \cdot \mathrm{s}$ and $k_{x}=160 \mathrm{~mol} / \mathrm{m}^{2} \cdot \mathrm{s}$. The equilibrium solubility data at $303 \mathrm{~K}$ are (Perry and Chilton, 1973)
Step by Step Answer:






