For mass transfer across the hollow-fiber membrane contactors described in Example 2.14, the overall mass-transfer coefficient based
Question:
For mass transfer across the hollow-fiber membrane contactors described in Example 2.14, the overall mass-transfer coefficient based on the liquid concentration, $K_{L}$, is given by (Yang and Cussler, 1986)
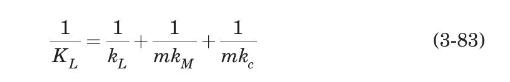
where $k_{L}, k_{M}$, and $k_{c}$ are the individual mass-transfer coefficients in the liquid, across the membrane, and in the gas, respectively; and $m$ is Henry's law constant, the gas equilibrium concentration divided by that in the liquid. The mass-transfer coefficient across a hydrophobic membrane is estimated from (Prasad and Sirkar, 1988)

where $\mathrm{D}_{A B}=$ molecular diffusion coefficient in the gas filling the pores $\varepsilon_{M}=$ membrane porosity $\tau_{M}=$ membrane tortuosity $\delta=$ membrane thickness For the modules of Example 2.14, $\varepsilon_{M}=0.4, \tau_{M}=2.2$, and $\delta=25 \times 10^{-6} \mathrm{~m}$ (Prasad and Sirkar, 1988).
(a) Calculate the corresponding value of $k_{M}$.
(b) Using the results of part (a), Example 2.14, and Problem 2.27, calculate $K_{L}$, and estimate what fraction of the total resistance to mass transfer resides in the liquid film.
Data From Example 2.14:-
Boiler feed water (BFW) must be deaerated to avoid corrosion problems in the boilers. Hollow fibers made of microporous polypropylene can be used for extremely fast removal of dissolved oxygen from water, therefore making a compact BFW deaerator possible (Yang and Cussler, 1986). For a given boiler, 40,000 kg/h of BFW is needed. Design a hollow-fiber membrane module for that purpose, if the unit can remove 99% of the dissolved oxygen present in natural waters at 298 K.
Data From Problem 2.27:-
(a) Consider the hollow-fiber BFW deaerator described in Example 2.14. If only oxygen diffuses across the membrane, calculate the gas volume flow rate and composition at the lumen outlet. The water enters the shell side at 298 K saturated with atmospheric oxygen, which means a dissolved oxygen concentration of 8.38 mg/L.
(b) Calculate the mass-transfer coefficient at the average conditions inside the lumen. Neglect the thickness of the fiber walls when estimating the gas velocity inside the lumen.
Step by Step Answer:






