(a) Calculate the enthalpy of formation of the hypothetical compound KF 2 assuming a CaF 2 structure....
Question:
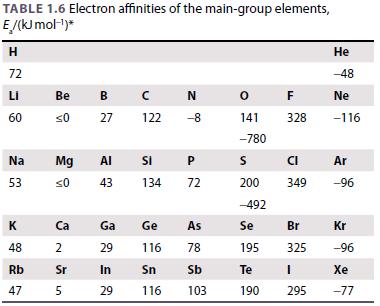
(a) Calculate the enthalpy of formation of the hypothetical compound KF2 assuming a CaF2 structure. Use the Born–Mayer equation to obtain the lattice enthalpy and estimate the radius of K2+ by extrapolation of trends in Table 1.4 and Resource section 1. Ionization enthalpies and electron gain enthalpies are given in Tables 1.5 and 1.6.
(b) What factor prevents the formation of this compound despite the favourable lattice enthalpy?
Table 1.4.
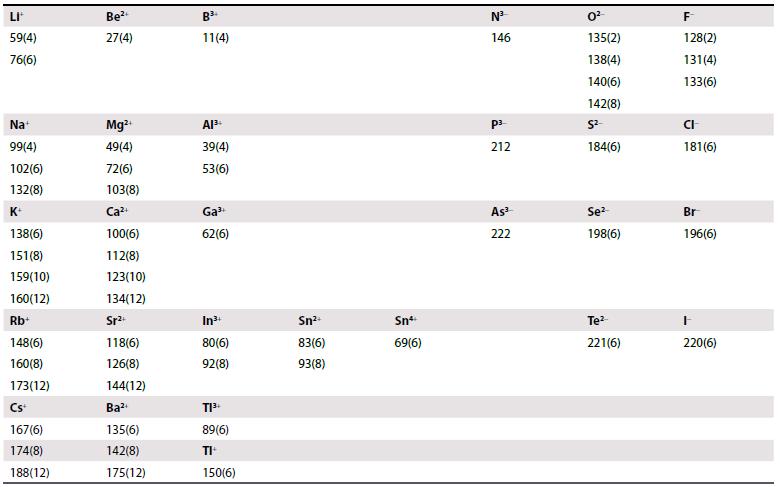
Table 1.5.
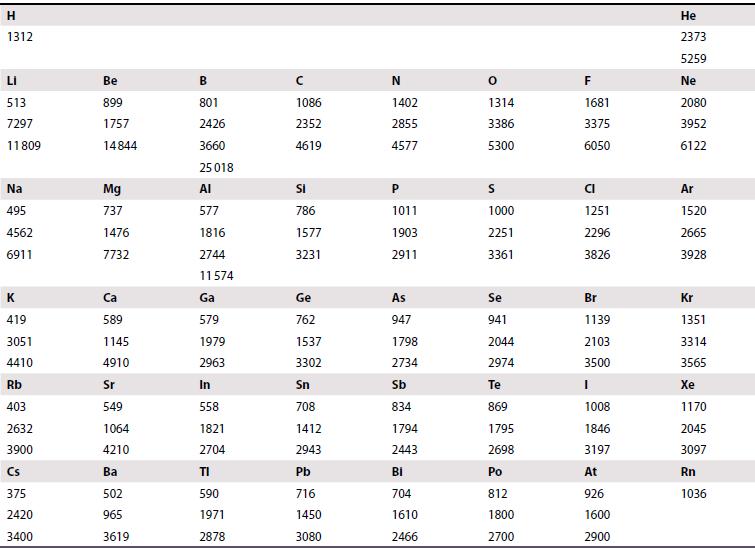
Table 1.6.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





