(a) Comment on the fact that, of the group 1 cations, Li + is the most strongly...
Question:
(a) Comment on the fact that, of the group 1 cations, Li+ is the most strongly solvated in aqueous solution, even though the first coordination shell only contains four H2O molecules compared with six for each of the later members of the group.
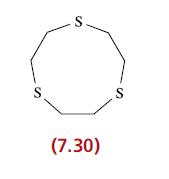
(b) Suggest how ligand 7.30 coordinates to Ru2+ in the 6-coordinate complex [Ru(7.30)2]2+. How many chelate rings are formed in the complex?
(c) For [Au(CN)2]‾, the stability constant K ≈ 1039 at 298 K. Write an equation that describes the process to which this constant refers, and calculate ΔGº(298 K) for the process. Comment on the magnitude of the value you obtain. This cyanide complex is used in the extraction of gold from its ore using the reactions:
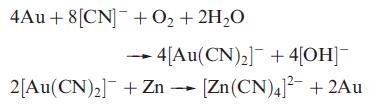
What processes take place in this extraction process?
Step by Step Answer:





