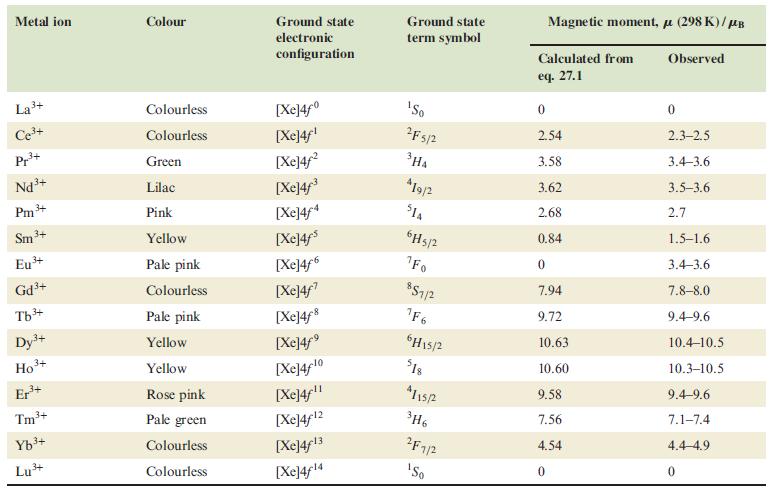
(a) Table 27.3 lists the calculated value of eff for Eu 3+ as 0. On what...
Question:
(a) Table 27.3 lists the ‘calculated’ value of μeff for Eu3+ as 0. On what basis is this value calculated? Explain why observed values of μeff for Eu3+ are greater than zero.
(b) The complex UO2Cl2(THF)3 contains one labile THF ligand and readily forms a diuranium complex, A, that contains 7-coordinate U(VI) with trans-UO2 units. A is a precursor to a number of mononuclear complexes. For example, one mole of A reacts with four moles of K[O-2,6-tBu2C6H3] to give two moles of B, and with four moles of Ph3PO eliminating all coordinated THF to yield two moles of C. Suggest identities for A, B and C and state the expected coordination environment of the U(VI) centre in each product.
Table 27.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





