(a) The ligand 1,4,7-triazacyclononane, L, forms the nickel complexes [NiL 2 ] 2 [S 2 O 6...
Question:
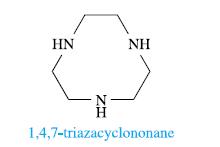
(a) The ligand 1,4,7-triazacyclononane, L, forms the nickel complexes [NiL2]2[S2O6]3·7H2O and [NiL2][NO3]Cl·H2O. X-ray diffraction data for these complexes reveal that in the cation in [NiL2][NO3]Cl·H2O, the Ni—N bond lengths lie in the range 209–212 pm, while in [NiL2]2[S2O6]3·7H2O, two Ni—N bonds (mutually trans) are of length 211 pm and the remaining Ni—N bonds are in the range 196–199 pm. Rationalize these data.
(b) Suggest why some reports of the properties of low-spin [Fe(bpy)3]2+ state that its salts possess very low magnetic moments.
(c) The ligand HL can be represented as follows:
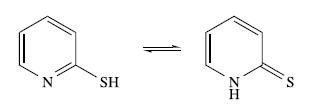
What is the term given to these forms of HL? The conjugate base of HL forms the complexes mer-[VL3]− and [V(Me2NCH2CH2NMe2)L2]. Draw the structure of mer-[VL3]−, and the structures of the possible isomers of [V(Me2NCH2CH2NMe2)L2].
Step by Step Answer:






