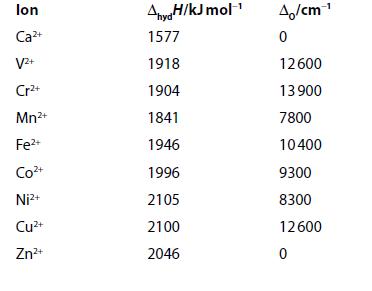
Enthalpies of hydration and values of the ligand-field splitting parameter, O , are given for some
Question:
Enthalpies of hydration and values of the ligand-field splitting parameter, ΔO, are given for some octahedrally coordinated ions.
(a) Plot the enthalpies of hydration against the number of d electrons.
(b) Calculate LFSE in terms of ΔO for high-spin configuration. Use the given values of ΔO to find LFSE in kJ mol−1 for each ion.
(c) Apply this energy as a correction term to the enthalpies of hydration and plot the estimated ΔH in the absence of ligand-field effects. Comment on this plot. (1 kJ mol−1 = 83.7 cm−1.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





