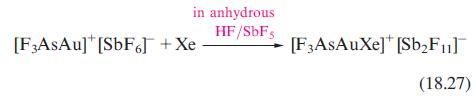
Equation 18.27 showed the preparation of [F 3 AsAuXe][Sb 2 F 11 ] from [F 3 AsAu][SbF
Question:
Equation 18.27 showed the preparation of [F3AsAuXe][Sb2F11] from [F3AsAu][SbF6]. Solid [F3AsAu][SbF6] contains a distorted [SbF6]− ion; one Sb–F bond is 193 pm, and five are in the range 185–189pm. The Au centre interacts with the F atom of the long Sb–F bond (Au–F = 212pm, compared with 203 pm calculated for the hypothetical [AuF2]− ion). Suggest why [F3AsAu][SbF6] was chosen as the precursor to [F3AsAuXe]+, rather than a route involving reduction of AuF3 in anhydrous HF/SbF5 in the presence of Xe.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





