Given the following standard potentials in basic solution and assuming that a reversible reaction can be established
Question:
Given the following standard potentials in basic solution
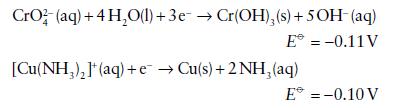
and assuming that a reversible reaction can be established on a suitable catalyst, calculate E⦵, ΔrG⦵, and K for the reductions of
(a) CrO42−
(b) [Cu(NH3)2]+ in basic solution. Comment on why ΔrG⦵ and K are so different between the two cases despite the values of E⦵ being so similar.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





