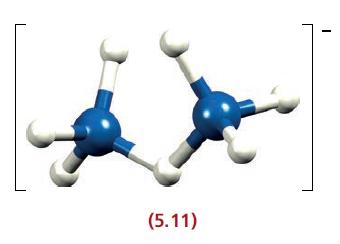
In [B 2 H 7 ] (5.11), each B atom is approximately tetrahedral. (a) How many valence
Question:
In [B2H7]‾ (5.11), each B atom is approximately tetrahedral.
(a) How many valence electrons are present in the anion?
(b) Assume that each B atom is sp3 hybridized. After localization of the three terminal B—H bonds per B, what B-centred orbital remains for use in the bridging interaction?
(c) Following from your answer to part (b), construct an approximate orbital diagram to show the formation of [B2H7]‾ from two BH3 units and H‾. What does this approach tell you about the nature of the B—H—B bridge?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





