In Problem 7.12 we looked at the successive formation constants for 1,2-diaminoethane complexes of three different metals.
Question:
In Problem 7.12 we looked at the successive formation constants for 1,2-diaminoethane complexes of three different metals. Using the same data, discuss the effect of the metal on the formation constant. How might the Irving–Williams series provide insight into these formation constants?
Data from Problem 7.12.
The equilibrium constants for the successive reactions of 1,2-diaminoethane with Co2+, Ni2+, and Cu2+ are as follows:
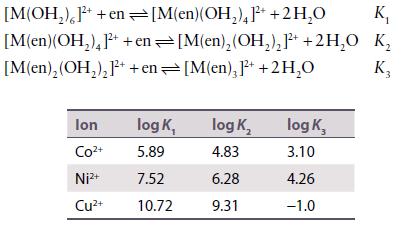
Discuss whether these data support the generalizations in the text about successive formation constants. How do you account for the very low value of K3 for Cu2+?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





