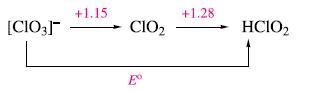
The following potential diagram is part of that illustrating the redox chemistry of chlorine in aqueous solution
Question:
The following potential diagram is part of that illustrating the redox chemistry of chlorine in aqueous solution at pH0.
(a) Calculate the value of E° for the reduction of [ClO3]‾ to HClO2.
(b) Justify why, in this case, the value of E° can simply be taken to be the mean of +1.15 and +1.28 V.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





