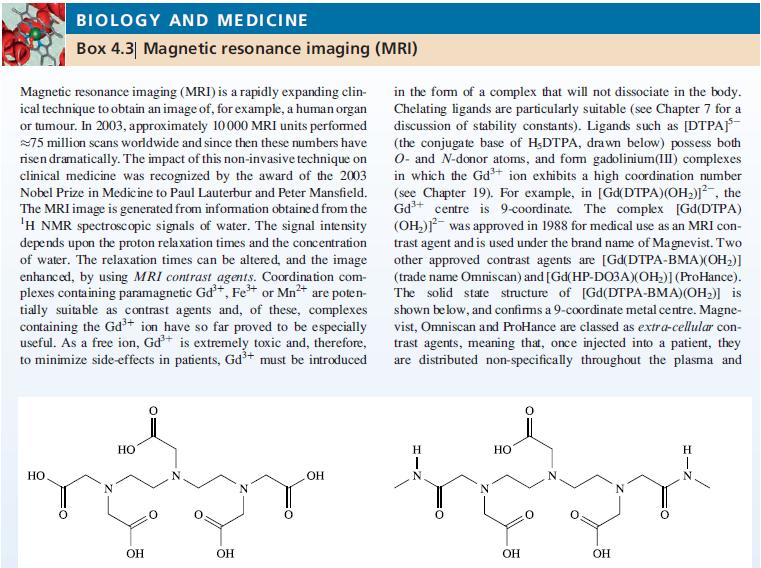
The structure of H 5 DTPA (see Box 4.3) is shown below: (a) Write equilibria to show
Question:
The structure of H5DTPA (see Box 4.3) is shown below:
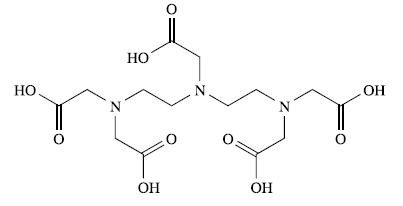
(a) Write equilibria to show the stepwise acid dissociation of H5DTPA. Which step do you expect to have the largest value of Ka?
(b) In the complex [Gd(DTPA)(OH2)]2−, the Gd3+ ion is 9-coordinate. Draw a diagram that illustrates how the DTPA5− ion binds to the metal centre in this complex. How many chelate rings are formed?
(c) Values of log K for the formation of [M(DTPA)]n+ complexes in aqueous media are as follows: Gd3+, 22.5; Fe3+, 27.3; Ag+, 8.7. Comment on these data.
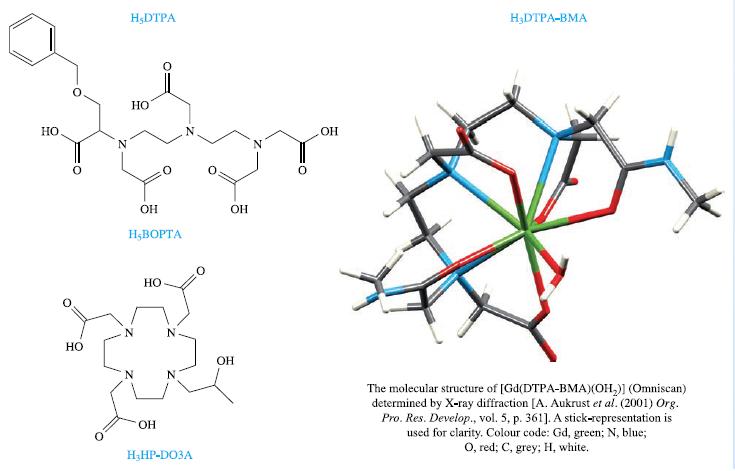
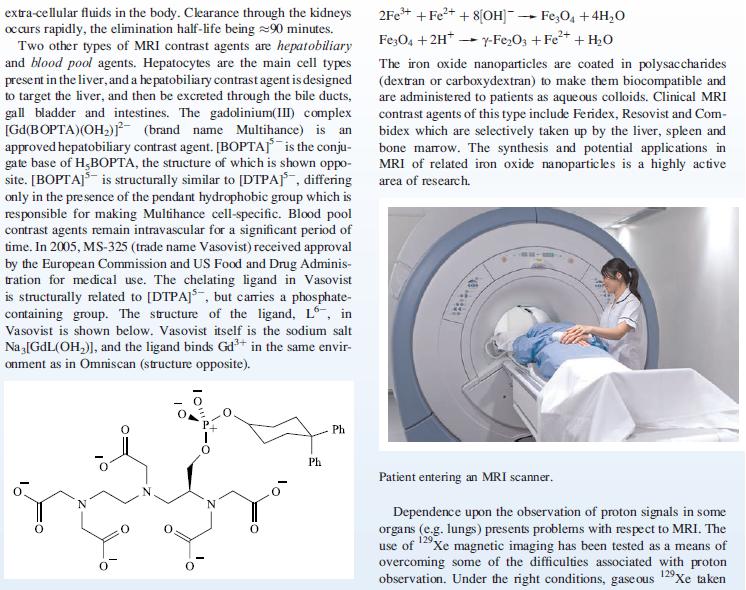
Box 4.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





