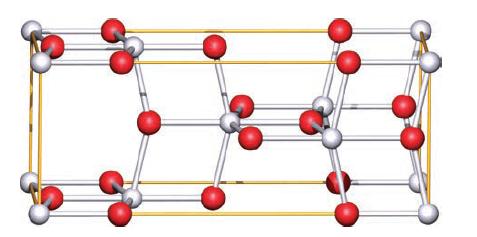
TiO 2 occurs naturally as three polymorphs: rutile (Fig. 6.24), anatase (Fig. 6.32) and brookite. (The unit
Question:
TiO2 occurs naturally as three polymorphs: rutile (Fig. 6.24), anatase (Fig. 6.32) and brookite. (The unit cells of rutile and anatase can be viewed in 3D by going to www.pearsoned.co.uk/housecroft and following the links to the figures.) Rutile is commercially important as a white pigment in paints, paper and plastics, and in the last stages of its manufacture, seed crystals of rutile are added. Anatase acts as a photocatalyst and has a vital role in, for example, dye-sensitized solar cells.
(a) What analytical method is routinely used to screen polymorphs of a compound? Explain briefly how the technique can be used to distinguish anatase and rutile.
(b) What are the coordination environments of each Ti(IV) centre and O2− ion in anatase?
(c) Use the unit cell in Fig. 6.32 to confirm the stoichiometry of the anatase phase.
(d) Anatase preferentially crystallizes under ambient conditions and transforms to rutile at higher temperatures. At the structural level, how do you envisage the phase change taking place?
Figure 6.24
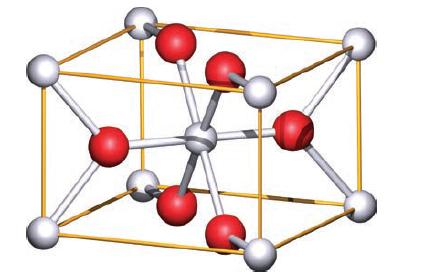
Figure 6.32
Step by Step Answer:






