Use the data in Table 14.2 and the additional bond enthalpy data given here to calculate the
Question:
Use the data in Table 14.2 and the additional bond enthalpy data given here to calculate the enthalpy of hydrolysis of CCl4 and CBr4. Bond enthalpies/kJ mol−1: O–H = 463, H–Cl = 431, H–Br = 366.
Table 14.2.
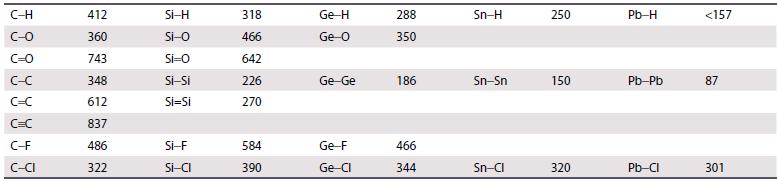
Transcribed Image Text:
C-H C-O C=0 C-C C=C C=C C-F C-CI 412 360 743 348 612 837 486 322 Si-H Si-O Si=O Si-Si Si=Si SI-F Si-CI 318 466 642 226 270 584 390 Ge-H Ge-0 Ge-Ge Ge-F Ge-Cl 288 350 186 466 344 Sn-H Sn-Sn Sn-Cl 250 150 320 Pb-H Pb-Pb Pb-Cl <157 87 301
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To calculate the enthalpy of hydrolysis of CCl4 and CBr4 we need to consider the breaking of CX X Cl ...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Use Table 8.4 to estimate the enthalpy change for each of the following reactions: a. H2C == O (g) + HCl (g) H3C - O - Cl (g) b. H2O2 (g) + 2CO (g) H2 (g) + CO2 (g) (c). 3H2C == CH2 (g) C6H12 (g)...
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
The "plastic" explosive C-4, often used in action movies, contains the molecule cyclotrimethylenetrinitramine, which is often called RDX (for Royal Demolition eXplosive):...
-
Project P costs $15,000 and is expected to produce benefits (cash flows) of $4,500 per year for five years. Project Q costs $37,500 and is expected to produce cash flows of $11,100 per year for five...
-
The Customer Service Department of Bragg Inc. asked the Publications Department to prepare a brochure for its training program. The Publications Department delivered the brochures and charged the...
-
Read the article from InTheBlack below and answer the questions below. New pathways to business success As you may know, our 20092011 corporate plan established the importance of having a globally...
-
Explain several implications of IFRS on financial reporting by health care organizations.
-
Two accountants for the firm of Allen and Wright are arguing about the merits of presenting an income statement in a multiple-step versus a single-step format. The discussion involves the following...
-
What will be the output from the following code? void myFunction(int &b){ } b = 1; int main(){ int a = 0; cout < < a < < " "; myFunction(a); cout < < a; return 0; }
-
Discuss the solid-state chemistry of silicon in silicates with reference to how the various structures can be built up from SiO 4 tetrahedra linked into polymeric anions, chains, rings, sheets, and...
-
The lightest p-block elements often display different physical and chemical properties from the heavier members. Discuss the similarities and differences by comparison of: (a) The structures and...
-
How active are US and EU firms in acquiring companies throughout the world? What accounts for this activity?
-
The vapor pressure of n-pentane is given by In Pbar = 8.630-2819.7/T + (1.855 10)T. Derive an expression for AHvap as a function of temperature and find AHvap at its normal boiling point 36.1 C.
-
Assume that consumers view haircuts as the same among sellers and there are hundreds of barbers in a given market. The current market equilibrium price for a haircut is $15. Bobs Barbershop has a...
-
Explain the main principles of critical thinking and describe how these might apply to individual and work colleagues ideas to assist objective and rationale debate.
-
2. a. What is the area under the normal curve between z = -1.0 and z = -2.0? b.The standard normal probability distribution is unique because it has ________.
-
Remote teams yes, prove to add different issues of their own due to the nature of not being solely located in a FTF format for staff to work through any issues. But, at the same time, are these...
-
Exercise 2 refers to a t test. What is a t test? Why are the t test methods of Part 1 in this section so much more likely to be used than the z test methods in Part 2?
-
What are the main distinctions between the different schools of legal interpretation?
-
Why is metal substitution used to investigate the metal binding site in carbonic anhydrase? Discuss the type of information that might be forthcoming from such a study.
-
Discuss the role of Zn 2+ as an example of a Lewis acid at work in a biological system.
-
(a) What is the function of cytochrome c oxidase? (b) Describe the four active metal-containing sites in cytochrome c oxidase and the proposed way in which they work together to fulfil the role of...
-
What accounts for the shifting emphasis over decades on private and public sector engagement in the US space industry?
-
What is an insurance policy's grace period? Explain.
-
Identify at least three unique risks Apple faces and how these risks will influence the audit strategy and procedures?

Study smarter with the SolutionInn App


