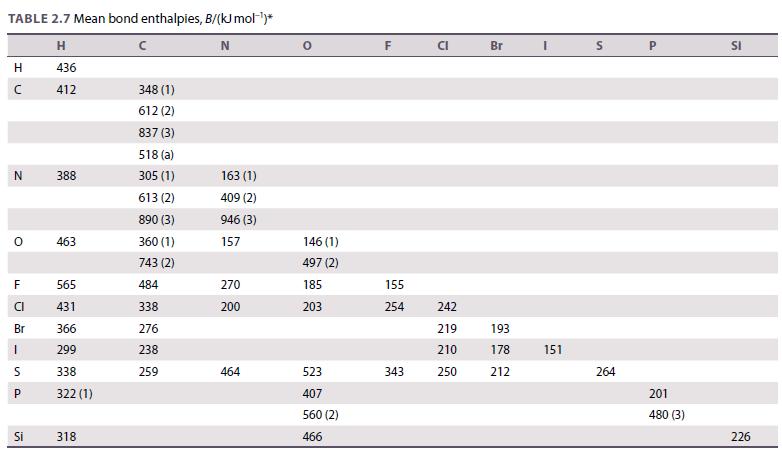
Use the bond enthalpy data in Table 2.7 to calculate the enthalpy of formation for NF 3
Question:
Use the bond enthalpy data in Table 2.7 to calculate the enthalpy of formation for NF3 and NCl3. Explain why NF3 is thermodynamically stable whereas NCl3 is unstable and reactive.
Table 2.7.
Transcribed Image Text:
TABLE 2.7 Mean bond enthalpies, B/(kJ mol-¹)* H N 436 412 H с N O F LL 565 431 Br 366 1 299 338 322 (1) U S P 388 Si 463 318 с 348 (1) 612 (2) 837 (3) 518 (a) 305 (1) 613 (2) 890 (3) 360 (1) 743 (2) 484 338 276 238 259 163 (1) 409 (2) 946 (3) 157 270 200 464 0 146 (1) 497 (2) 185 203 523 407 560 (2) 466 F 155 254 343 CI 242 219 210 250 Br 193 178 212 I 151 S 264 P 201 480 (3) SI 226
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the enthalpy of formation for NF3 and NCl3 using bond enthalpy data we need to use the following equation Hf Hbonds broken Hbonds formed ...View the full answer

Answered By

Akash M Rathod
I have been utilized by educators and students alike to provide individualized assistance with everything from grammar and vocabulary to complex problem-solving in various academic subjects. I can provide explanations, examples, and practice exercises tailored to each student's individual needs, helping them to grasp difficult concepts and improve their skills.
My tutoring sessions are interactive and engaging, utilizing a variety of tools and resources to keep learners motivated and focused. Whether a student needs help with homework, test preparation, or simply wants to improve their skills in a particular subject area, I am equipped to provide the support and guidance they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Explain why an epoxide is a relatively stable product, whereas a bromonium ion is a reactive intermediate.
-
Suppose that two firms are competing in a Stackelberg (sequential quantity setting). How would forming a cartel affect the profits of each firm? (Hint: make up a linear inverse demand function and...
-
In arm wrestling, there are four muscles primarily used :Biceps:brachii: Pronator teres: Pectoralis major: Flexor carpi ulnas Other muscles such as the Deltoid latissimus dorsii and theTriceps...
-
Write a detailed executive summary about the appropriation and advancement of Information and Communication Technology (ICT)
-
Presented below is information related to Lexington Real Estate Agency. Oct. 1 Diane Lexington begins business as a real estate agent with a cash investment of $20,000 in exchange for common stock. 2...
-
Find a parametric representation for the surface. The plane that passes through the point (0, -1, 5) and contains the vectors (2, 1, 4) and (-3, 2, 5)
-
In July 2017, Latrice Merritt entered a residential lease with Doran 610 Apartments, LLC. Under the terms of the lease agreement, Merritt was prohibited from installing a private security system in...
-
The air pollution project discussed in the chapter has progressed over the past several weeks, and it is now the end of week 8. Lester Harky would like to know the value of the work completed the...
-
Simplify the given expression and write the answer with only positive exponents. -113 P 3/7 7 3 54x y z 2 w) 3) (w + 2) (3w + w 4 2 (-3x +7x+8)= (x * + 7x *-12x-1) - 5 27-7-28 20-x-22 712-24-8...
-
P. Christiansen et al. describe Relativistic effects in chemical systems in their 1985 paper (Annu. Rev. Phys. Chem., 2001, 36, 407). How did they define relativistic effects? Briefly summarize the...
-
Use the following data to calculate average values of B(SeF) in SeF 4 and SeF 6 . Comment on your answers in view of the corresponding values for B(S-F) in SF, (+340 kJ mol-) and SF a (+329kJmol-):...
-
Use the data contained in Figure 1.6 and Figure 1.10 to calculate productivity in terms of output per $ spent on labor for the countries listed in both figures. Which five countries are the most...
-
Describe how you would collaborate with the child's family to implement the short-term goal and strategies you've identified. These are my short term goals: Short-term goal: Learn to recognize the...
-
Can someone please help me with Dynamic Binding. This is my 5th time trying to get a program running but I have been late due to personal issues. I wanted to make a dynamic binding program based on a...
-
1. Cherry Auto Sales just opened and does not expect to pay a dividend during its first year. At the end of its second year, Cherry's owners expect to pay a $2.00 dividend and plan to increase it 7%...
-
One assumption of the case is that were the hotel to outsource laundering of banquet linen, the cost they would incur per tablecloth would be directly linked to the amount of banquet linen laundered....
-
If the GBP/USD rate is 1.3450, how much GBP can $1 million USD buy? 1. 743,494 GBP 2. 1,345,000 GBP 3. 1,340,000 GBP 4. 863,976 GBP
-
Which of the following sets of quantum numbers are not allowed? For each incorrect set, state why it is incorrect. a. n = 3, = 3, m = 0, ms = - 1/2 b. n = 4, = 3, m = 2, ms = - 1/2 c. n = 4, = 1,...
-
Explain why it is not wise to accept a null hypothesis.
-
HSO is an intermediate in the atmospheric oxidation of H 2 S, and it has been implicated in ozone depletion. Calculated wavenumbers for the fundamental modes of vibration of HSO are 2335, 1077 and...
-
Suggest products for the following reactions (which are not balanced): (a) AgCI+ CIF3 (b) CIF + BF3 (c) CsF+IFs - (d) SbF5 + CIF5 (e) Me NF + IF A (f) K[BrF4]
-
The reaction of TeCl 4 with PPh 3 in THF solution in air leads to the formation of the salt (Ph 3 PO) 2 H] 2 [Te 2 Cl 10 ]. Structural data reveal that each Te centre in the anion is in an...
-
what is it when undeposited cash should be stored in the company safe? Explain.
-
Discuss which types of engagements, other than a traditional audit, require the auditor to be independent and which do not. Explain why independence is important regardless of the requirement for it.
-
Jorge and Anita, married taxpayers, earn $150,000 in taxable income and $40,000 in interest from an investment in City of Heflin bonds. Using the U.S. tax rate schedule for married filing jointly,...

Study smarter with the SolutionInn App


