Question: Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H2(g) + O2(g) → 2 H2O(g). The experimental value is −484 kJ. Account for the difference between the estimated and experimental values.
Table 2.7.
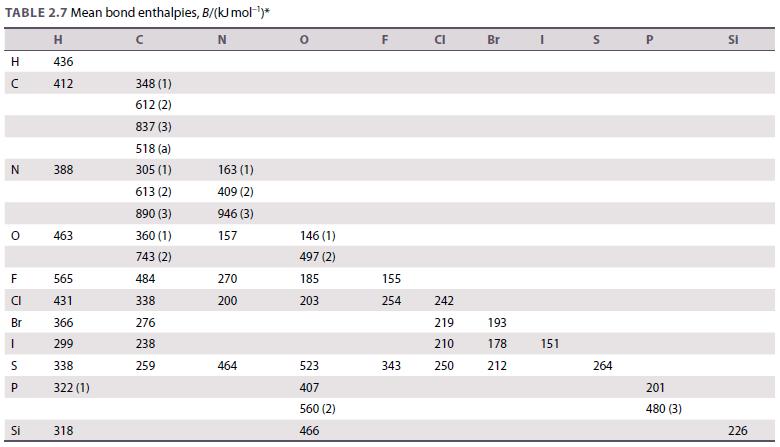
TABLE 2.7 Mean bond enthalpies, B/(kJ mol-)* H N 436 412 H N O F CI Br LL 1 S P Si 388 463 565 431 366 299 338 322 (1) 318 348 (1) 612 (2) 837 (3) 518 (a) 305 (1) 613 (2) 890 (3) 360 (1) 743 (2) 484 338 276 238 259 163 (1) 409 (2) 946 (3) 157 270 200 464 146 (1) 497 (2) 185 203 523 407 560 (2) 466 F 155 254 343 CI 242 219 210 250 Br 193 178 212 I 151 S 264 P 201 480 (3) SI 226
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
To calculate the standard enthalpy of the reaction 2 H2g O2g 2 H2Og using the data in Table 27 we will use Hesss Law which states that the overall ent... View full answer

Get step-by-step solutions from verified subject matter experts


