Activity coefficients are an implicit part of the equation of state but they can be determined explicitly
Question:
Activity coefficients are an implicit part of the equation of state but they can be determined explicitly by comparing the definitions of the K-ratios. Using the kij value fit at xe = 0.415, compute the activity coefficients implied by the Peng-Robinson equation for the benzene + ethanol system and compare them with the UNIFAC values and the values determined from the experimental data of Brown and Smith (1954) cited in problem 10.2, using plots of activity coefficient versus composition.
Data from problem 10.2:
Benzene and ethanol (e) form azeotropic mixtures. Prepare a y-x and a P-x-y diagram for the benzene-ethanol system at 45°C assuming the mixture is ideal. Compare the results with the experimental data tabulated below of Brown and Smith, Austral. J. Chem. 264 (1954). (P in the data table is in bar.)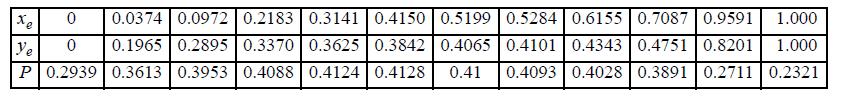
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





