Beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent
Question:
Beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent Gibbs energy of formation of ADP at the conditions of Example 18.10, using the extended Debye-Hückel activity coefficient model and transformed Gibbs energies. Also calculate the distribution of each species.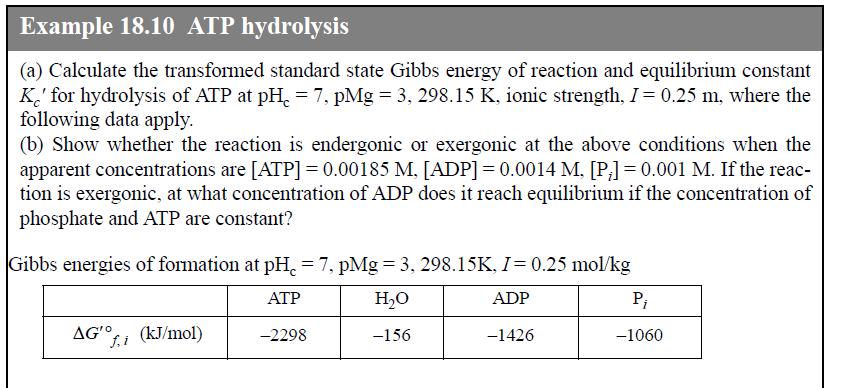
Transcribed Image Text:
Example 18.10 ATP hydrolysis (a) Calculate the transformed standard state Gibbs energy of reaction and equilibrium constant K' for hydrolysis of ATP at pH=7, pMg = 3, 298.15 K, ionic strength, I = 0.25 m, where the following data apply. (b) Show whether the reaction is endergonic or exergonic at the above conditions when the apparent concentrations are [ATP] = 0.00185 M. [ADP] = 0.0014 M, [P;] = 0.001 M. If the reac- tion is exergonic, at what concentration of ADP does it reach equilibrium if the concentration of phosphate and ATP are constant? Gibbs energies of formation at pH = 7, pMg = 3, 298.15K, I = 0.25 mol/kg ATP H₂O ADP -2298 -156 -1426 AG'O f.i (kJ/mol) P₁ -1060
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Repeat problem 18.20, but use H 3 PO 4 . Data from problem 18.20 Beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent Gibbs...
-
At pH c = 7, I = 0.25, beginning with the untransformed Gibbs energies of formation, document the intermediate calculations for the value of apparent Gibbs energy of formation of CO 2 at the...
-
The first step in biological glycolysis (the catabolic reaction for glucose consumption) involves addition of a phosphate to create glucose 6-phosphate 2 . If the reaction were to occur in aqueous...
-
Mr. Silkwallah established the Fashion Clothing Company (FCC) to market designer clothes. The business was to get designer clothes produced by tailors, exclusively for FCC. FCC provides the following...
-
Man Demko is the loading dock supervisor for a dry cement packaging. His work crew is composed of unskilled workers who load transport trucks with bags of cement, gravel, and sand. The work is hard,...
-
A taxpayer studies tax law at a university on a part-time basis. He is diligently working towards a master's degree in taxation. He is employed on a full-time basis in the auditing department of a...
-
Air at \(300 \mathrm{~K}\) and \(1 \mathrm{~atm}\) flows along a flat plate at \(3 \mathrm{~m} / \mathrm{s}\). At a location of \(0.3 \mathrm{~m}\) from the leading edge, find the thickness of the...
-
Each Triam Deluxe computer system consists of two speakers, a monitor, a system unit, a keyboard, and an installation kit. These pieces are packed together and shipped as a complete kit. In MRP...
-
Draw the Logic Diagram for the following Boolean expressions. a) F = (x'y + y'z' )(x+ y') (b) F A+BZ+A'B'Z'
-
Repeat problem 18.18, but use ADP. Data from problem 18.18 (a) Write the binding polynomial for ATP at 298.15K in terms of binding constants in the absence of Mg for application between 3 < pH c <...
-
(a) Write the binding polynomial for ATP at 298.15K in terms of binding constants in the absence of Mg for application between 3 < pH c < 14. Assume ideal solutions. (b) Convert the binding constants...
-
ValuMart, a large national retail chain, is nearing its fiscal year-end. It appears that the company is not going to hit its revenue and net income targets. The companys marketing manager, Chris...
-
Suppose TCPs measured RTT is 1.0 except that every Nth RTT is 4.0. What is the largest N, approximately, that does not result in timeouts in the steady state (i.e., for which the Jacobson/Karels...
-
In the following list, you will find pairs of individuals. For each pair, check your ASSIGNMENT 6.2 state code and answer three questions: Can the individuals marry? If the marriage is prohibited, is...
-
Show that the Internet checksum can be computed by first taking the 32-bit ones complement sum of the buffer in 32-bit units, then taking the 16-bit ones complement sum of the upper and lower...
-
a. Is your state statutory code on the Internet? If so, give its address (uniform resource locator or URL) and quote from any statute on this site that covers divorce. b. Try to find the Internet...
-
Predict the solubility (in mole fraction) of phenol at the cited conditions using the specified model. (i) Use the MAB model. (ii) Use the SSCED model. (iii) Use the UNIFAC model. (a) Solubility in...
-
Some overhead data for Pine Company are given in BE. In addition, the flexible manufacturing overhead budget shows that budgeted costs are $4 variable per direct labor hour and $50,000 fixed. Compute...
-
Using Apple, demonstrate how the differentiation strategy can be well implemented.
-
Obtain the surface and contour plots for the function z = -x 2 + 2xy + 3y 2 . This surface has the shape of a saddle. At its saddlepoint at x = y = 0, the surface has zero slope, but this point does...
-
Suppose that y = x 2 , where x is a normally distributed random variable with a mean and variance of x = 0 and 2 x = 4. Find the mean and variance of y by simulation. Does y = 2 x ? Does y = 2...
-
Suppose you have analyzed the price behavior of a certain stock by plotting the scaled frequency histogram of the price over a number of months. Suppose that the histogram indicates that the price is...
-
Adams Boards produces two kinds of skateboards. Selected unit data for the two boards for the last quarter follow: Basco Boards Shimano Boards Production costs Direct materials Direct labor Allocated...
-
Ending work in process units: 2,000 units * 60% = 1,200 units * 60% = 720 units Total equivalent units for conversion costs: 11,000 units + 720 units = 11,720 units why are you multiplying 60% twice...
-
Jennifer was self - employed and earned $ 2 7 0 0 of income. It is her only income for the year. Should she file a tax return. Yes, no and why?

Study smarter with the SolutionInn App


