Express the Joule-Thomson coefficient in terms of measurable properties for the following: (a) Van der Waals equation
Question:
Express the Joule-Thomson coefficient in terms of measurable properties for the following:
(a) Van der Waals equation given in Example 6.6
(b) An ideal gas.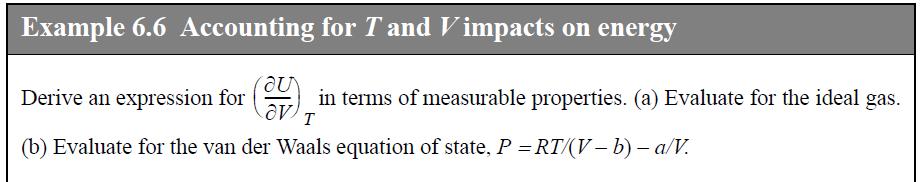
Transcribed Image Text:
Example 6.6 Accounting for T and Vimpacts on energy au Derive an expression for Cou (b) Evaluate for the van der Waals equation of state, P = RT/(V-b) - a/V. in terms of measurable properties. (a) Evaluate for the ideal gas. T
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The JouleThomson coefficient denoted by relates the change in temperature of a gas when it expands o...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Express the Joule-Thomson coefficient in terms of measurable properties for the following a. Vander Waals equation b. An ideal gas.
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
In Sample Exercise 10.16, we found that one mole of Cl2 confined to 22.41 L at 0oC deviated slightly from ideal behavior. Calculate the pressure exerted by 1.00 mol Cl2 confined to a smaller volume,...
-
(5 points) In a study of purchasing behavior at a small shop, it was found that the probabaty that a purchase is more than $5 is 0.29, the probability that a customer will pay with a credit card is...
-
Give an example of a negative externality and an example of a positive externality.
-
A company that operates 10 hours a day manufactures two products on three sequential processes. The following table summarizes the data of the problem: Determine the optimal mix of the two products....
-
Your colleague is excited about your good fortune (Problem 3.1) at work, but she only got the promise of a watch or \($300\) cash. You convince her that she will be better in the long run by just...
-
The treasurer of Westmark Industrial, Inc ., a wholesale distributor of household appliances, wants to estimate his company's cash balances for the first three months of 2018. Using the following...
-
Ashley and Duncan just created their January 2019 income and expense statement. They spent their combined $3,500 gross monthly income on the following expenses: $350 for tithing, $1,000 on rent, $400...
-
(a) Prove (b) For an ideal gas along an adiabat, (P/P i ) = (T/ T i ) C P /R . Demonstrate that this equation is consistent with the expression from part (a). JP OT S || P TVOp
-
Express the adiabatic compressibility, in terms of measurable properties. Ks || 1 S
-
Allison invests $15,000 in an account paying 7% per year compounded annually. (a) How many years are required for the compound amount to at least double? (b) In how many years will the amount at...
-
In what way may a tenant be in a difficult position when the premises burn down, even if the lease states that the rent is suspended until the premises are rebuilt?
-
What are the principal powers given to the board of directors of a corporation incorporated under the CBCA?
-
How is partnership property distributed on the dissolution of a partnership?
-
Describe the landlords duty to mitigate after a tenant abandons the premises.
-
How are directors appointed? How may they be removed?
-
Reread the chapter-opening vignette. 1. In your view, have the Steeles really planned for the future? Discuss your answer. 2. Realizing the information is limited indicate which of the familys goals...
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
A 40.0-mL solution of 0.040 0 M Hg 2 (NO 3 ) 2 was titrated with 60.0 mL of 0.100 M KI to precipitate Hg 2 I 2 (K sp = 4.6 10 -29 ). (a) Show that 32.0 mL of KI are needed to reach the equivalence...
-
Write the mass balance for CaCl 2 in water if the species are Ca 2+ and Cl - . (b) Write the mass balance if the species are Ca 2+ , Cl - , CaCl - , and CaOH + . (c) Write the charge balance for part...
-
(a) Write the mass balance for CaCl 2 in water if the species are Ca 2+ and Cl - . (b) Write the mass balance if the species are Ca 2+ , Cl - , CaCl - , and CaOH + . (c) Write the charge balance for...
-
Who has the better argument in Armstrong v Exceptional Child Center Inc , Scalia or Sotomayor, and why?
-
If you sold it for 4,000,000 the investment 6 years from now and received the $100,000 cash flow each of the next 6 years, what would your compound annual return actually be on this investment?
-
Students will read the "Evolution of Federal Interest" section in the textbook (13th Edition: Pages 236-239) and complete the following steps in their discussion post: 1. Provide a brief summary of...

Study smarter with the SolutionInn App


