Hydrogen gas can be produced by the following reactions between propane and steam in the presence of
Question:
Hydrogen gas can be produced by the following reactions between propane and steam in the presence of a nickel catalyst: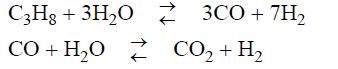
Neglecting any other competing reactions:
(a) Compute the equilibrium constants at 700 K and 750 K.
(b) What is the equilibrium composition of the product gas if the inlet to the catalytic reactor is propane and steam in a 1:5 molar ratio at each of the temperatures and 1 bar?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





